表观基因组生物标记、靶向治疗耐受机制;美国在研的甲状腺癌基金,给医生科学家(2024)
2024-10-23 Hanson临床科研 Hanson临床科研 发表于上海
我们仅对美国国立卫生研究院(NIH)资助的在研甲状腺癌相关项目进行梳理,希望给同仁们的选题思路提供一点启发。
甲状腺癌(Thyroid Cancer)是源自甲状腺组织的恶性肿瘤,它主要包括几种组织学类型,如乳头状、滤泡状、髓样和未分化甲状腺癌。乳头状甲状腺癌是最常见的类型,通常具有较好的预后。甲状腺癌的发展可能与遗传和环境因素如辐射暴露有关。
目前针对甲状腺癌的重要而未解决的临床问题主要集中在以下几个方面:
-
早期诊断与分子标志物的研究:虽然多数甲状腺癌预后良好,但早期诊断仍然是一个挑战,尤其是在无症状患者中。目前急需开发敏感且特异的分子标志物以提高早期诊断的准确性。
-
治疗耐药性问题:对于晚期或复发性甲状腺癌,现有治疗策略(包括放射性碘治疗、外科手术、放疗及靶向治疗)可能面临耐药性问题。特别是BRAF和RET突变驱动的甲状腺癌,在靶向治疗后容易发展出耐药性。
-
未分化甲状腺癌的治疗策略:未分化甲状腺癌(Anaplastic Thyroid Carcinoma, ATC)是一种高度恶性且预后极差的甲状腺癌类型。这类癌症对传统治疗反应差,迫切需要新的治疗方法和策略。
-
个体化治疗的发展:随着对甲状腺癌分子生物学的深入了解,发展个体化治疗方案成为可能。通过精准医疗,可以根据患者的具体基因突变和癌症特征来制定治疗方案,提高治疗的有效性和安全性。
-
长期随访与生活质量的改善:甲状腺癌患者在接受治疗后需进行长期随访以监控复发及远处转移,同时治疗相关的副作用对患者的生活质量有显著影响,如何在提高治疗效果的同时,改善患者的生活质量,也是当前研究的重点。
我们仅对美国国立卫生研究院(NIH)资助的在研甲状腺癌相关项目进行梳理,希望给同仁们的选题思路提供一点启发。
2024年,以“Thyroid Cancer”为检索词、在题目中进行检索,美国NIH针对甲状腺癌的在研有30项。
一,谁获得了这些研究?
1,在研甲状腺癌基金最多的PI
-
NATIONAL INSTITUTE OF DIABETES AND DIGESTIVE AND KIDNEY DISEASES 的 KLUBO-GWIEZDZINSKA, JOANNA
-
BECKMAN RESEARCH INSTITUTE/CITY OF HOPE 的 HAHN, MARIA
-
DIVISION OF BASIC SCIENCES - NCI 的 CHENG, SHEUE-YANN
-
DIVISION OF BASIC SCIENCES - NCI 的 BOUFRAQECH, MYRIEM
-
UNIVERSITY OF MICHIGAN AT ANN ARBOR 的 HAYMART, MEGAN RIST
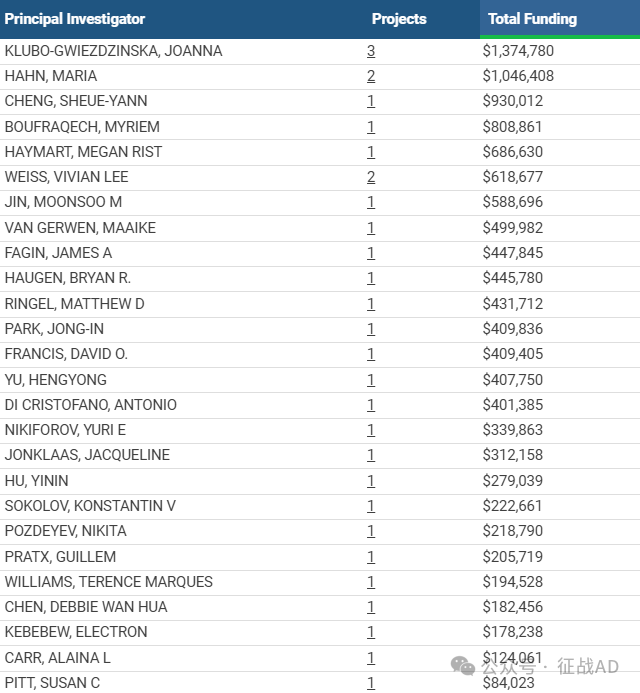
2,甲状腺癌基金最多的研究机构
-
基础科学部 - NCI
-
美国国家糖尿病、消化和肾脏疾病研究所
-
贝克曼研究所/希望之城
-
密歇根大学安娜堡分校
-
科罗拉多大学丹佛分校等
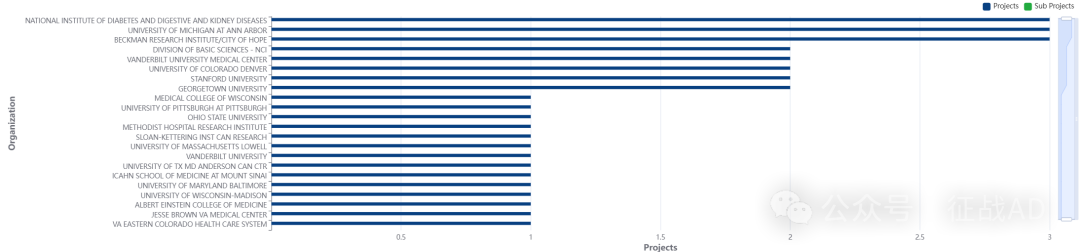
二,甲状腺癌研究热点是什么?
甲状腺癌研究领域总览(根据关键词)
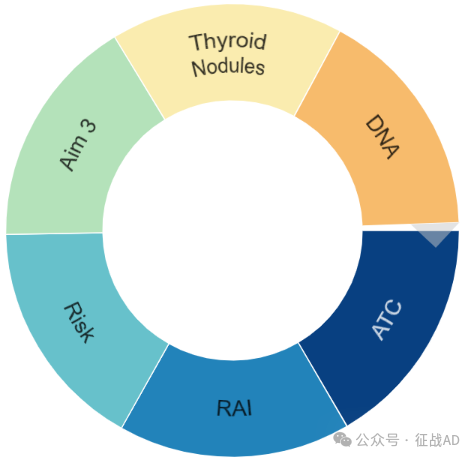
甲状腺癌大的研究方向包括ATC、RAI、风险(Risk)、DNA、甲状腺结节(Throid Nodules)等。
三,借鉴与突破
我们也分享在甲状腺癌领域的几项课题摘要,希望对同仁们有所启发。
A,Unlocking mechanisms of resistance to targeted therapy in thyroid cancer
Aim 1: Identify novel genes or essential pathways required for resistance to BRAFV600E inhibition utilizing genome wide and focused CRISPR-based screens. First, using BRAFV600E-ATC cell line 8505c, we will harness a human CRISPR knockout library of 19,050 genes to identify genes whose loss enhances cell death by the BRAF selective inhibitor Dabrafenib. Dabrafenib has a better toxicity profile compared to the canonical inhibitor vemurafenib and was recently approved by the FDA for the treatment of advanced and metastatic thyroid cancer. BRAF activity will be inhibited using 400 nM of Dabrafenib, a dose which yielded about 20% of cell death and a complete inhibition of phospho-MEK and phospho-ERK1/2 in a 14-days treatment survival assay. Mechanisms of resistance to targeted therapy including those involving BRAFV600E inhibitors is a complex process which involve various alterations that enable cancer cells to escape death. To more specifically unlock these mechanisms of resistance, we engineered a focused CRISPR/cas9 and CRISPR/dCas9 transcriptional activation (SAM system) libraries targeting genes sets selected based on strong correlations with BRAFV600E activation and dependency, in collaboration with the Genome modification Core of the NCI-Frederick. We will utilize single sgRNA CRISPR guides targeting top genes identified in our CRISPR screens output to validate the putative synthetic lethality with BRAFV600E inhibition. To further substantiate the significance of essential gene (s) in vivo, we will use two models 1) ATC cells 8505c will be depleted for the validated gene(s) and used in an immunocompromised xenograft model for ATC. 2) The murine BRAFV600E-driven ATC cell line will be depleted for the validated gene(s) and implanted orthotopically into immunocompetent mice. In both mouse models tumor growth and metastasis of wild type and target-depleted cells will be evaluated in response to dabrafenib. This study will identify novel genes involved in resistance to BRAFV600E inhibition and will unfold new avenues of therapeutic strategies in patients with anaplastic thyroid cancer.
Aim2: Targeting ATM and BRAFV600E in anaplastic thyroid cancer for precision medicine. Preclinical studies in cancer therapy have shown that targeting major genome maintenance genes such as ATM, ATR, or harnessing deficiency in DNA damage response genes such as BRCA1, may help to overcome drug resistance in multiple solid malignancies. These findings provide insights into targeting DNA repair proteins to sensitize cancer cells to targeted therapies. In line with these observations, we found that ATM expression is strongly associated with adverse clinical features and aggressive thyroid cancer histology, thereby suggesting that ATM may play a key role in mediating thyroid cancer metastasis, particularly in BRAF-driven thyroid tumors. ATM is a major genome maintenance gene involved in the repair of double-stranded DNA breaks. While several reports have demonstrated links between genetic alterations in ATM and DNA repair response genes, little is known about how changes in ATM expression level and kinase activity affect cancer progression. We aim to determine the extent to which ATM expression level is associated with key features of thyroid cancer aggressiveness, and whether ATM could serve as a druggable therapeutic target in advanced and metastatic thyroid cancer. To elucidate the role of ATM in thyroid cancer progression we performed functional studies and found that ATM is a key regulator of VEGF secretion and angiogenesis in ATC. Furthermore, ATM depletion or its inhibition with a highly selective ATM inhibitor reduced tumor metastasis in a model of ATC xenografts. On the other hand, little is known about the impact of BRAFV600E inhibition on DNA repair pathways in solid tumors. Our preliminary data show that increasing doses of BRAFV600E inhibitors dabrafenib (FDA approved) and PLX4720 (modified vemurafenib) lead to a robust phosphorylation of ATM on its Serine1981 residue in BRAFV600E driven ATC cells. These observations are consistent with two major recent findings demonstrating an increased phosphorylation of ATM in EGFR and BRAFV600E inhibitors treated NSCLC and melanoma cells respectively. Together, these data suggest that therapy targeting oncogenes such as BRAFV600E induces dependency on ATM activation in cancer cells, and combination of ATM inhibitor AZD1390 (currently in clinical trial) with specific BRAFV600E inhibitors such as dabrafrenib will unlock acquired resistance to BRAFV600E inhibitors in ATC. Remarkably, we found that AZD1390 sensitizes ATC and PDTC BRAFV00E driven cells to dabrafenib. The validation of the clinical benefit of this combination therapy in a genetically engineered ATC mouse model (BRAFV600E; Trp53R270H) with a similar phenotype and disease aggressiveness as in human patients, will provide a valuable new therapeutic strategy that may significantly improve the clinical management of patients with undifferentiated thyroid cancer.
B, Validation of epigenomic biomarkers for thyroid cancer diagnostics
Here, we will rigorously test and validate the ability of our epigenetic biomarkers to evaluate the biologic aggressiveness of thyroid nodules and determine whether the new epigenetic testing will improve thyroid nodule management towards the eradication of unnecessary thyroidectomies. Current molecular diagnostics for indeterminate thyroid nodules, while providing some improvement, have not eliminated the unnecessary thyroidectomies. Current molecular diagnostics are based on molecular differences between normal thyroid tissue and thyroid cancer. However, benign thyroid nodules can contain many molecular alterations including gene fusions and mutations. As a result, over half of thyroid nodules with a significant cancer risk according to the current molecular classifiers are found to be benign after thyroidectomy. In our published preliminary data, we performed a genome-wide DNA methylation analysis of 109 surgically excised thyroid nodules and adjacent benign tissue. We found that the DNA methylation pattern in benign nodules is different from thyroid cancer and normal thyroid. Based on the DNA methylation pattern specific to benign nodules and the DNA methylation pattern specific to thyroid cancer, we developed the Diagnostic DNA Methylation Signature (DDMS) approach to distinguish between benign versus malignant nodules. In a retrospective pilot study performed under 1R21CA223367, we developed DDMS further (DDMS-2). We tested the ability of the DDMS-2 assay to distinguish benign from malignant surgically excised thyroid nodules (n=121).
In this Pilot study, DDMS- 2 had an estimated positive predictive value (PPV) of 96% and a negative predictive value (NPV) of 98%. Guided by our preliminary data, we hypothesize that DDMS (i) can be successfully used for molecular thyroid cancer diagnostics of pre-operative thyroid nodule aspirations; (ii) will have superior performance in comparison to current thyroid cancer molecular testing and (iii) can affect physician decision-making towards elimination of unnecessary thyroidectomies. We will accomplish our overall objective by pursuing the following specific aims: Aim 1: To perform analytical validation of the DDMS-2 assay. Aim 2: To determine the DDMS-2 accuracy in a prospective cohort obtained from 7 medical centers and containing 1450 thyroid nodule aspirations including 800 aspirations with indeterminate cytopathology. Aim 3: To compare the diagnostic accuracy between DDMS- 2 and two current thyroid cancer molecular diagnostic approaches and to evaluate how the knowledge of the DDMS-2 results impacts clinical management of thyroid nodules. The development of a more accurate assay to distinguish benign and malignant thyroid nodules will address current clinical limitations and reduce the number of needless thyroidectomies and associated morbidities.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言












#甲状腺癌# #生物标记#
15