常见病如何申请基金?美国在研的322项心力衰竭研究给你启发(2024)
2024-10-28 Hanson临床科研 Hanson临床科研 发表于上海
我们仅对美国国立卫生研究院(NIH)资助的在研心力衰竭相关项目进行梳理,希望给同仁们的选题思路提供一点启发。
心力衰竭(Heart Failure),又称为充血性心力衰竭,是一种严重的临床症状复合体,其中心脏无法有效地泵血以满足身体的需求。这种情况可能是由心脏病、高血压、心脏瓣膜疾病等多种心血管疾病引起的。
尽管近年来在心力衰竭的诊断和治疗方面取得了显著进展,但仍存在一些重要而未解决的临床问题:
-
早期诊断:心力衰竭的早期诊断仍然具有挑战性,因为早期症状可能与其他疾病相似或不够明显。
-
治疗反应个体差异:患者对现有治疗方法的反应存在个体差异,如药物治疗和装置治疗(如心脏再同步治疗)。
-
长期管理:心力衰竭患者需要持续的监测和治疗调整,以优化治疗效果和改善生活质量,但有效的长期管理策略尚未完全确定。
-
先进治疗方法的开发:虽然药物和手术等治疗已取得进展,但治疗心力衰竭的新方法(如干细胞治疗和基因治疗)仍处于研究阶段。
-
复发和住院:减少心力衰竭患者的复发率和降低因心力衰竭导致的再次住院率是当前临床上亟需解决的问题。
心力衰竭的研究和治疗是一个动态发展的领域,需要跨学科的合作和创新以改善患者的预后和生活质量。
我们仅对美国国立卫生研究院(NIH)资助的在研心力衰竭相关项目进行梳理,希望给同仁们的选题思路提供一点启发。
2024年,以“Heart Failure”为检索词、在题目中进行检索,美国NIH针对心力衰竭的在研有322项。
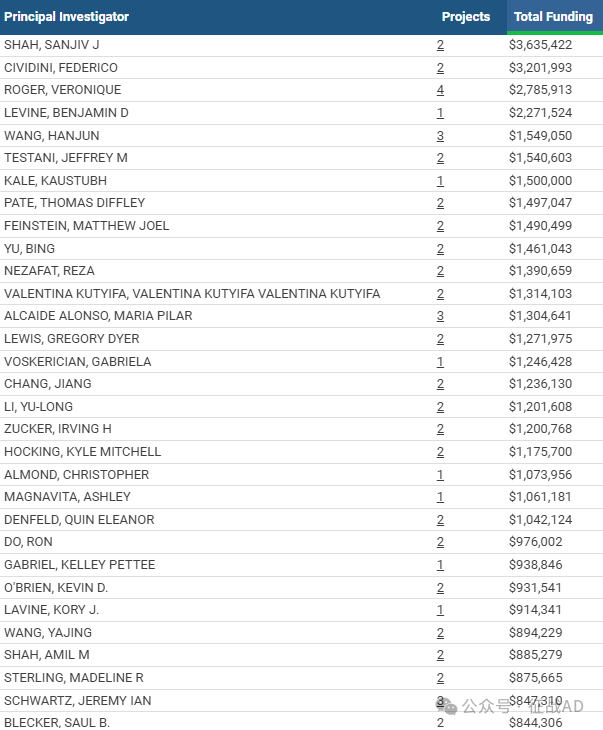
一,谁获得了这些研究?
1,在研心力衰竭基金最多的PI
-
NORTHWESTERN UNIVERSITY AT CHICAGO 的 SHAH, SANJIV J
-
CARDIAC RSK3 INHIBITORS, LLC 的 CIVIDINI, FEDERICO
-
NATIONAL HEART, LUNG, AND BLOOD INSTITUTE 的 ROGER, VERONIQUE
-
UT SOUTHWESTERN MEDICAL CENTER 的 LEVINE, BENJAMIN D
-
UNIVERSITY OF NEBRASKA MEDICAL CENTER 的 WANG, HANJUN
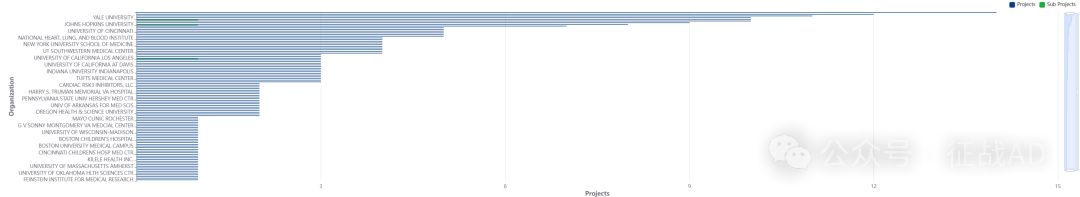
2,心力衰竭基金最多的研究机构
-
芝加哥西北大学
-
杜克大学
-
麻省总医院
-
内布拉斯加大学医学中心
-
耶鲁大学等
二,心力衰竭研究热点是什么?
心力衰竭研究领域总览(根据关键词)
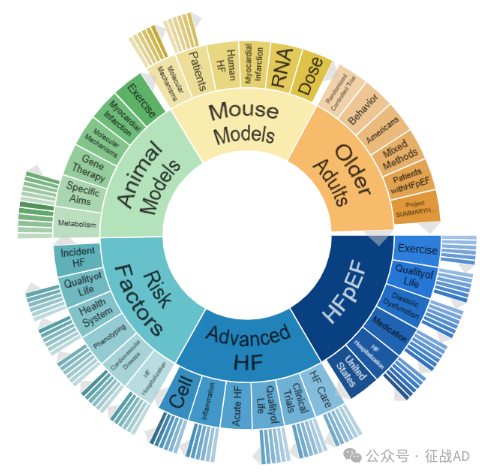
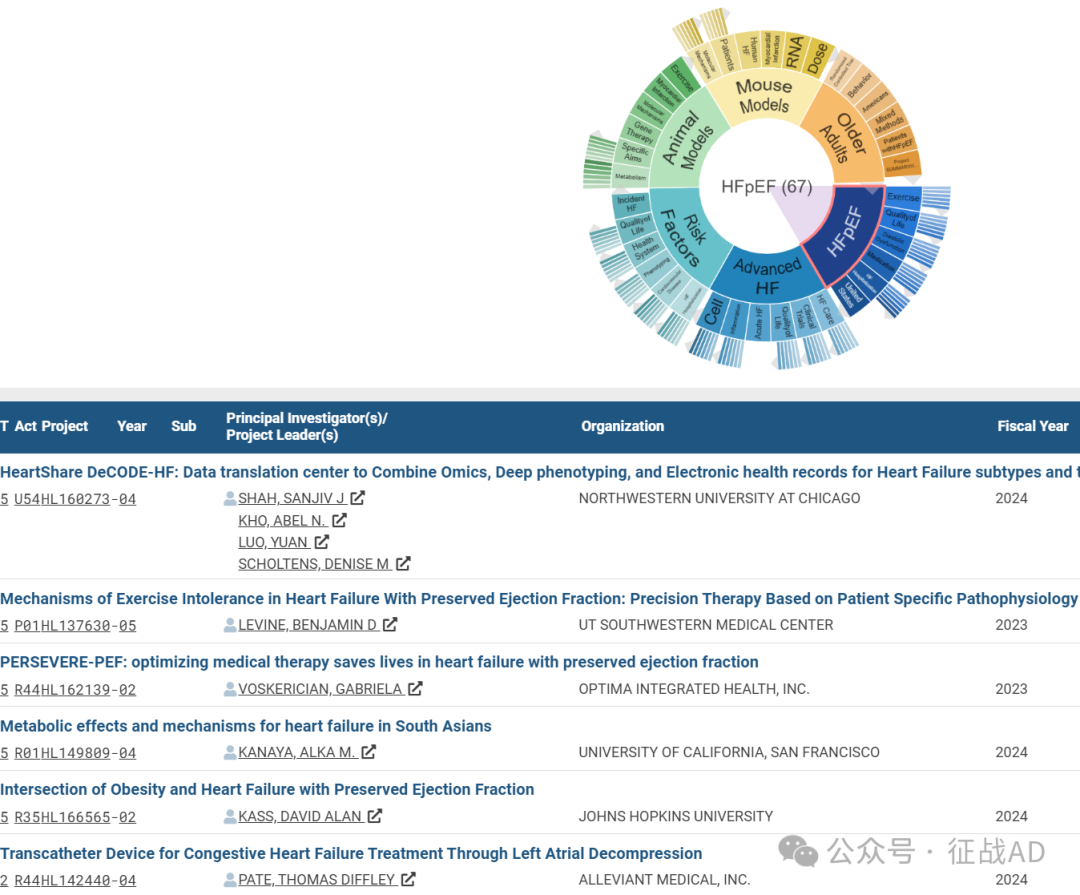
A,关于射血分数保留的心力衰竭(HFpEF)的研究项目最多
有 67 项在研基金涉及到了射血分数保留的心力衰竭,关注最多的方面包括锻炼(Exercise)、生活质量、舒张功能障碍(Diastolic Dysfunction)、药物治疗(Medication)、HF住院治疗(HF Hospitalization)等方面研究。
B,晚期心力衰竭(Advanced HF)的研究
有 57 项研究涉及到晚期心力衰竭,研究领域主要涉及HF治疗(HF Care)、临床研究(Clinical Trials)、生活质量、急性心衰(Acute HF)、炎症(Inflammation)、细胞(Cell)等方面研究。

C,风险因素(Risk Factors)
有 47 项研究涉及到风险因素,涉及的关键词包括HF住院治疗(HF Hospitalization)、心血管疾病(Cardiovascular Disease)、表型(Phenotyping)、保健系统(Health System)、生活质量、突发性心力衰竭(Incident HF)等。
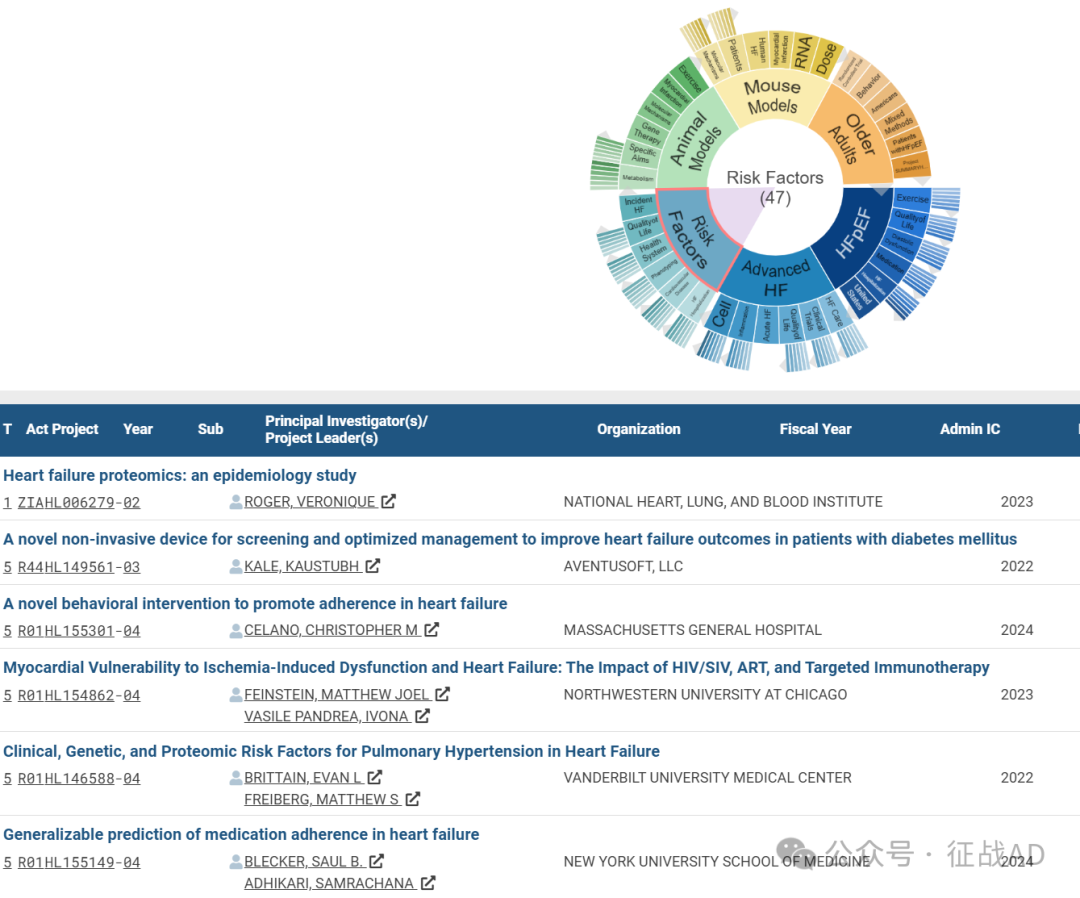
其他心力衰竭研究大的方向也包括动物模型(Animal Models)、小鼠模型(Mouse Models)、老年人(Older Adults)等。
三,借鉴与突破
我们也分享在心力衰竭领域的几项课题摘要,希望对同仁们有所启发。
A,HeartShare DeCODE-HF: Data translation center to Combine Omics, Deep phenotyping, and Electronic health records for Heart Failure subtypes and treatment targets
The Overarching Aim of the Northwestern HeartShare DeCODE-HF: Data translation center to Combine Omics, Deep phenotyping, and Electronic health records for Heart Failure subtypes and treatment targets is to provide overall management and oversight for HeartShare, including coordination and communication across all subps and cores of the program, and with the 4 HeartShare Clinical Centers (CCs). The Data Translation Center (DTC) will need to ensure timely completion of the retrospective and prospective components of HeartShare. In our application, we demonstrate the ability and prior experience of our multi-PI team and core leaders in the conduct and leadership of large-scale, multi-center studies, particularly in the realms of heart failure with preserved ejection fraction (HFpEF), electronic health record (EHR)-based investigation, deep phenotyping, machine learning, and biorepositories.
For both the retrospective and prospective HeartShare components, the Northwestern DTC will leverage its considerable experience in: (1) cohorts and trials that have data on adjudicated HF patients, and (2) its leadership and track record as the top enroller in HFpEF trials/studies to ensure optimal recruitment by the HeartShare CCs.
We will also leverage our expertise in HFpEF, data coordination/management, BioData Catalyst, EHR-based research, biostatistics, machine learning, multi-omics, human-computer interaction, and mobile health data monitoring to (1) successfully execute the HeartShare program and (2) meet the HeartShare DTC goals of providing a rich resource to the research community to advance the science of next-generation phenomics to identify HFpEF subtypes and therapeutic targets.
Our HeartShare DTC will carry out each of its 4 primary responsibilities. (1) The Administrative and Outreach Core will oversee HeartShare program operations, research skills development, and capitation to CCs for deep phenotyping costs; support a call center to follow patient outcomes; and promote rapid and broad data-sharing of clinical and molecular data. (2) The Data Portal Core will serve as the primary access point (via a web-based interface) and coordinating center for all HeartShare data; and to develop an interactive patient-facing web-based interface for remote consent, completion of forms, and collection of mobile health data using BioData Catalyst tools. (3) The Data Management Core will oversee ensure data quality, integration and harmonization of various data types; perform advanced analytics; coordinate biospecimen and imaging biorepositories; and integrate with TOPMed for omics analyses. (4) The Cohort Core will aggregate data on HF patients from completed epidemiology cohorts and HF trials and will combine data with further bioprofiling of existing samples to allow discovery/validation of novel targets.
B, Mechanisms of Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Precision Therapy Based on Patient Specific Pathophysiology
(Fleg 2015) We propose to address each of the working group recommendations through a unique PPG that links 4 projects and 3 cores to address the global objective of determining the mechanisms of exercise intolerance and dyspnea in patients with HFpEF and based on this pathophysiology, designing cre- ative exercise interventions that improve quality of life. Although virtually all Program Project Grants are linked together by a common theme, this PPG will be unique in that the individual projects will be linked even more closely by working together on common patients. All patients will be recruited into the Program by a specializ- ed Recruitment Core; each patient will then undergo high resolution physiological phenotyping by all 4 projects followed by a tailored intervention designed to test the clinical relevance of the phenotyping strategy.
This approach will allow us to accomplish the following specific aims: Specific Aim 1: To determine the mechan- ism(s) of exercise intolerance and DOE in patients with HFpEF using invasive and non-invasive cardio- pulmonary exercise testing. Patients will be divided into those with a primarily “central” limitation and those with a primarily “peripheral limitation” based on this initial assessment. Specific Aim #2: to determine whether a “precision” exercise intervention based on the high resolution phenotyping will lead to clinically meaningful improvements in functional capacity and reduction in dyspnea.
All patients will be randomized to undergo one of two tailored exercise interventions for 16 weeks: a) small muscle mass, single leg kicking exercise to allow high intensity muscle exercise without challenging cardiovascular reserve; b) reduce cardiac filling pressure during exercise with acute ingestion of TNG to allow higher systemic workloads and improved training.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言















#心力衰竭# #美国国立卫生研究院#
19