【协和医学杂志】主动脉夹层后血压管理的研究进展
2024-09-27 协和医学杂志 协和医学杂志 发表于上海
本文主要就AD对血压的影响、AD后血压管理的意义及方式进行综述,以期为AD患者的治疗提供参考。
综 述
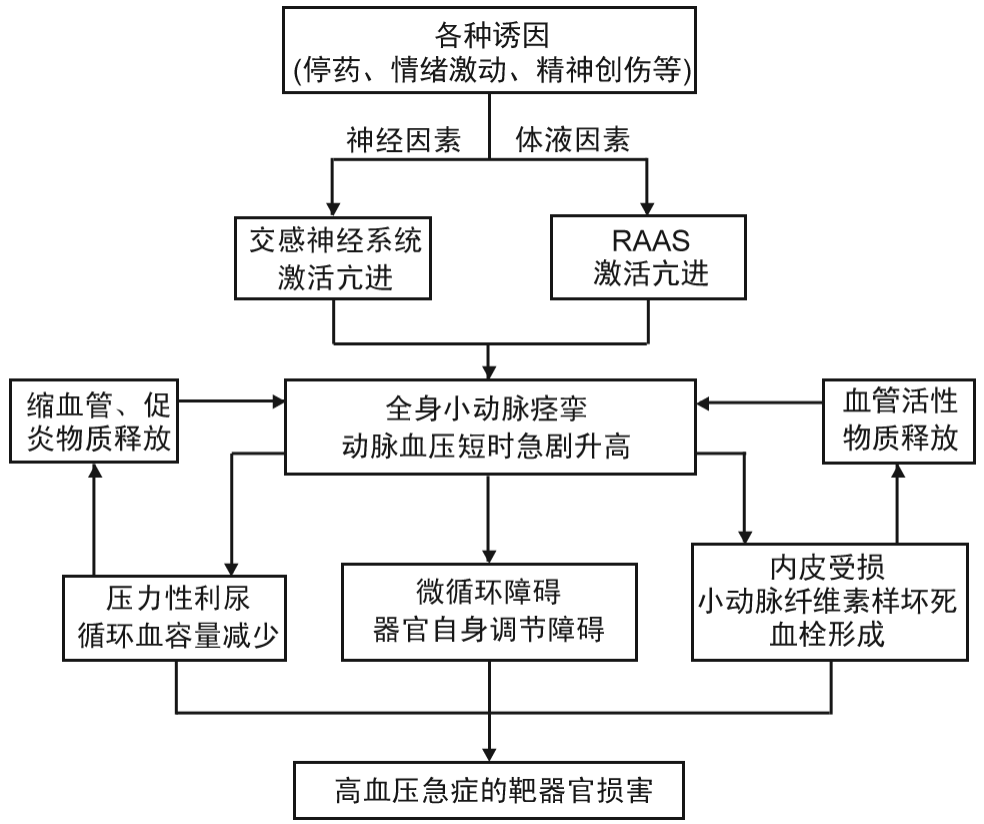
高血压(HT)通过增加主动脉管壁张力和促使组织结构退化而诱发主动脉夹层(AD),其与AD呈正相关且存在分级关联现象,在一般人群中收缩压每升高20 mm Hg(1 mm Hg=0.133 kPa),舒张压每升高10 mm Hg,AD相关死亡风险加倍[1-2]。HT诱发AD的机制具有独立性,与其他危险因素无关,因此AD也是高血压急症之一[3-5]。
另一方面,持续的HT将增加主动脉相关死亡风险,是AD预后的显著影响因素,也是最重要的可控因素,AD后血压管理可降低AD复发率及死亡率[1,6],因此控制HT是AD治疗的重要目标和手段。本文主要就AD对血压的影响、AD后血压管理的意义及方式进行综述,以期为AD患者的治疗提供参考。
1 AD对血压的影响
1.1 直接影响
主动脉内膜撕裂形成的AD根据破裂口所在部位及累及范围分为Stanford A型、B型,DebakeyⅠ~Ⅲ型等不同类型,AD发生后通过多种机制影响机体的血压调节。约75%~80%的急性主动脉夹层(AAD)患者存在HT,其中Stanford B型AAD患者较A型发生HT的比例更高(80.9%比74.4%,P<0.001)[7]。
在使用≥3种降压药物(包括利尿剂)后,收缩压仍不能得到良好控制者被定义为顽固性高血压(又称抵抗性高血压/耐药性高血压,RHT),而使用降压药物≥5种者为难治性高血压(RfHT),但目前这两种概念仍存在界定不清的问题[1,8]。研究表明,一般高血压患者RHT患病率约为16%[9],而AD患者中比例更高,约64%的AAD患者发生RHT,甚至部分患者在AD发病前血压正常[10]。
Eggebrecht等[3]研究发现,即使在长期随访的慢性AD患者中,RHT患病率仍为40%。Koracevic等[11-12]也认为大多数AD患者存在RHT且控制不佳。虽受用药方案、诊断标准等因素影响,不同时期数据存在一定差异,但从临床实践及显著的数据差异可以推断AD发病对血压有较大影响。
1.2 间接影响
虽然AD间接影响血压的机制尚不明确,但计算机断层扫描血管造影(CTA)显示,AD常累及肾动脉[13]。肾灌注受损会影响肾素血管紧张素醛固酮系统(RAAS),进而间接影响血压。而CTA无法评估动态肾灌注受损情况,以及肾动脉旁内膜瓣运动对肾灌注产生的可能影响[14]。采用肾动脉造影对161例AD患者肾灌注情况进行评估发现,83%的AD患者存在肾灌注不良,而采用CTA评估肾灌注不良的灵敏度、特异度仅为65%、58%[15]。因此,仅采用CTA检查存在低估肾灌注受损以及对远期血压的影响问题。
此外,国内外研究表明,近半数AD患者伴有精神障碍,约1/5的A型AD患者伴有创伤后应激障碍综合征,其中女性和低教育程度者患病率更高[16-17],这会导致内分泌和器官功能障碍,并可能诱发AD后的交感神经激活,从而引起HT及RHT[18]。动物实验表明,在心理应激状态下大鼠的肾上腺结构和功能会发生改变[19]。虽然目前研究证据尚不充分,但可推测AD能够通过心理-神经内分泌途径间接影响患者血压。
此外,Reed等[20]研究发现,部分AD患者发病前的血压控制较好甚至正常,但患病后HT严重程度增加,因此认为主动脉剥离过程对血压的调节具有深远而持久的影响,需进一步研究阐明AD间接影响血压的病理机制,如血流动力学、神经内分泌等诸多因素。
2 AD后血压管理的意义
Nienaber等[6]在一项全球性研究中发现,血压与AD的发病率和死亡率呈正相关,若能将收缩压降至120 mm Hg以下,理论上可将AD发病率与死亡率降低50%以上。
一项前瞻性研究表明,HT是导致AAD患者死亡的主要危险因素,收缩压和舒张压在病情平稳(低破裂风险且无分支灌注障碍)患者的一级预防中具有至关重要的作用[21]。
Kimura等[22]对1001例Stanford A型AD患者的病因与远期预后的相关性进行分析发现,以单纯HT为病因者占比71.13%(712/1001)。亚组分析显示,以单纯HT为病因者在年龄>45岁和≤45岁组的占比均较高,分别为71.70%(651/908)、65.60%(61/93)。即使在非HT病因组也有较高比例的患者合并HT(17%~64%),年轻急性Stanford A型AD患者的主要病因仍为HT,且术后10年长期生存率较其他病因组更低。HT的发病率极高,而AD的发病率相对较低,因此AD并非HT人群的必然转归,HT也非AD的单一危险因素。然而,HT会增加心脑血管事件和靶器官损害的风险,是AD发生发展的危险因素。控制HT是AD治疗的重要目标和手段,也是最易控制的致病因素[23]。同时识别并评估其他致病因素,将更有助于准确判断AD患者的预后。
血压一段时期内的波动性变化趋势称为血压变异性(BPV),常用收缩压的标准差来量化。Song等[24]研究认为,对于Stanford B型AAD患者而言,BPV的诊治价值高于HT,同时也是影响AD预后的重要因素之一。
Zhang等[25]研究发现,无论HT控制如何,高BPV会显著影响胸主动脉腔内修复术(TEVR)后假腔血栓化并增加AD患者主动脉相关死亡率(28.4%比9.1%, P=0.001),且在 RHT和非RHT亚组分析中均发现,高BPV会导致更高的主动脉相关死亡率(41.4%比14.3%,P=0.023;20.0%比7.0%,P=0.037)。虽然BPV与心血管疾病相关,但其难以被准确检测和比较,这也导致血压管理中的BPV重视不足或其判断具有较大的主观性。近年来,无袖带可穿戴血压监测技术的应用可获取目标群体自然状态下血压的连续变化情况,有望解决上述难题[26],并为后期BPV与AD的相关性研究奠定基础。
一般来说,HT会使动脉壁的弹力纤维受损,分子修复机制下调。而降低血压的脉压(即血管内张力)可保护动脉弹力纤维,维持动脉壁稳定。因此,对于任何年龄段或是否存在动脉性疾病的HT患者而言,有效平稳的血压管理均非常重要[27]。就AD患者而言,相当一部分患者在行腔内或开放手术治疗后仍残留部分夹层,主动脉也存在更多的薄弱区,此类人群的血压管理更加重要。AD后的血压应控制在最低可耐受范围,且不增加脑梗死、心肌梗死等缺血性心血管事件的发病率,然而对于伴有RHT和RfHT患者而言,这一目标较难实现[28]。
3 AD后血压管理的方式
3.1 调整生活方式
血压管理的基础是坚持健康的生活方式(包括健康的饮食习惯、体质量控制、戒烟、避免酗酒、有氧运动等)[1-2,29-30]。一项针对53 397例HT患者的前瞻性研究发现,健康生活方式组罹患心脏代谢性疾病的风险较不健康生活方式组降低41%,特定心血管疾病及糖尿病发病率降低32%~50%,其中不吸烟最为重要,对心血管的保护作用也最强[31]。此外,调整生活方式对RHT也有良好的控制作用,Blumenthal等[32]研究发现,坚持4个月的饮食调节和运动可显著降低RHT患者的临床和动态血压,且心血管疾病相关指标均得到改善。
对于成人和青少年而言,肥胖(特别是腹型肥胖)易导致HT,减重并坚持采用终止高血压膳食疗法(坚持食用水果蔬菜、动植物优质蛋白及低糖、低脂、低盐的饮食方式)有助于控制血压[33]。此外,阻塞性睡眠呼吸暂停综合征(OSAS)与肥胖密切相关,而肥胖和OSAS均是RHT的独立危险因素[1-2]。OSAS导致的缺氧与HT及AD等心脑血管疾病密切相关[34],而由OSAS引起的一系列疾病(包括交感神经系统过度活跃、心率变异性升高、血压升高、心肌壁应激、氧化应激、全身炎症、血小板聚集和血管内皮功能受损等)与心血管和神经体液等复杂病理机制有关,由此进展为长期且严重的HT甚至RHT,是AD等心血管疾病发生发展和预后不良的重要因素,同时也影响AD患者的长期血压管理效果[35]。坚持使用持续气道正压通气(CPAP) 虽可起到一定的降压作用,改善心血管疾病相关指标(证据水平A),减少心血管事件发生(证据水平B)[36],但无法改善OSAS患者的肥胖问题,只有通过减重才能减少OSAS患者HT、糖尿病等心血管疾病的发生(证据水平C)。
Iida等[37]研究认为,老年人的食盐摄入量和体质量主要影响收缩压,减少食盐摄入可增强RAAS抑制剂的降压效果[38]。如摄入富含钾、镁的蔬菜水果则有利于降低血压,保护心血管[1,39]。此外,饮酒对心血管系统的影响较为复杂,长期饮酒会引起血压升高或减弱血压管理效果、干扰交感神经功能、通过多种机制间接损伤血管内皮[40],上述生活方式均需引起AD患者的重视。
3.2 药物治疗
对于AD患者而言,长期规律使用降压药物,将血压降至最低可耐受水平极为重要。除指南推荐用于治疗HT的一线药物噻嗪类利尿剂、血管紧张素转换酶抑制剂(ACEI)、血管紧张素受体拮抗剂(ARBs)和钙通道阻滞剂(CCBs)外[1],β受体阻滞剂也可用于AD治疗,该类药物能降低AD患者主动脉壁的剪切应力,改善远期生存率[41-42]。上述治疗药物除通过血压相关因素改善AD预后外,也可能通过其他途径影响AD发病与进展[3,43]。
Tehrani等[44]研究表明,低于降压治疗剂量的缬沙坦可显著减缓马凡综合征患者主动脉根部的扩张,但目前尚无研究证实与β受体阻滞剂相比,ARBs治疗AD效果更好[45]。β受体阻滞剂与ACEI的联合治疗可对交感神经系统和RAAS发挥互补性作用,降低心血管疾病风险并改善预后[46]。由于RAAS对血压调控、心血管重塑及肾功能调节的作用机制复杂,除经典的RAAS拮抗剂ARBs和ACEI外,针对该系统其他成分及其受体作为治疗靶点的药物研究也取得了诸多进展,或可为AD患者提供更多的治疗选择。以氢氯噻嗪为代表的利尿剂是治疗RHT、RfHT的常规药物,而螺内酯除作为利尿剂外,也在RHT治疗中发挥拮抗盐皮质激素受体这一重要作用[47-48]。
然而,多数患者采用单药治疗无法达到降压目的,需进行多药联合治疗,但这一方式会导致患者依从性降低[28,49-50],这也是目前慢病防治中普遍存在的问题。据统计,HT患者1年内服用多种降压药的依从率仅为50%[51],而AD患者因随访率较高,降压治疗依从性稍好,其在7.1年中位随访期内的降压治疗依从率可达64%[42],Stanford B型AD患者既往接受手术治疗也可显著提高其降压治疗依从率[52]。加深患者对疾病的认识,采用个体化指导是提高依从性的有效方法。此外,通过简化给药方案、优化剂型,也能明显提高患者依从性[1,46]。
3.3 手术治疗
Usai等[53]研究发现,在RHT和非RHT患者中,Stanford B型AD患者在行主动脉夹层腔内修复术后平均收缩压和舒张压显著降低,分析原因可能是支架植入能显著改善主动脉分支的管腔重塑,对于真腔供血分支的改善尤为明显。这将明显改善包括肾脏在内的脏器与肢体的血流灌注,从而有助于降低血压[54]。
Wang等[55]对1353例患者进行分析发现,减重术可显著降低收缩压和舒张压,对年龄>45岁、体质量指数(BMI)<40 kg/m2的非重度肥胖、腰围<115 cm的患者降压效果较好。这一方式也可降低运动相关高血压(EIH),尤其适用于部分女性患者,无论术前是否患有高血压,肥胖患者减重术后2周平均动脉压均有所降低[56-57]。
OSAS作为HT和RHT的致病因素之一,与其相关的鼻/喉科手术对血压管理也有一定作用。112例OSAS合并HT患者在行OSAS相关手术后,收缩压和舒张压均显著降低[(146±15.3)mm Hg降至(122±12.5)mm Hg;(91±10.2)mm Hg降至(76±7.8)mm Hg],其中51.8%的患者术后不再需要口服降压药[58]。Lim等[59]认为,应将能否降低HT相关事件作为评价此类手术成败的标准。虽然OSAS相关手术对降低心血管疾病风险有一定作用,但相关临床指南认为目前尚缺乏足够令人信服的文献支持,仅作为D级推荐[36]。
肾交感神经消融术(RSD) 用于治疗RHT,通过降低肾交感神经及中枢交感神经活性来降低血压[60-62]。由于AD患者的预后更多依赖于对术后血压的有效管理,RSD可使AD合并RHT的患者从中获益[63]。Divchev等[64]对6例DebakeyⅢB型AD伴严重RHT患者采用RSD治疗,降压效果较好且无手术相关并发症。
另有研究表明,RSD不仅可降低静息血压,也可降低运动或环境压力相关HT,且不影响血压昼夜曲线,这对于AD合并RHT的年轻患者回归正常生活及改善预后更具积极意义[65-66]。此外,RSD还有助于改善患者的焦虑、抑郁等不良情绪[67]。但RSD治疗效果不稳定[68],目前仍不能作为HT治疗的常规手段,仅用于对血压管理需求更为迫切的患者群体。
3.4 免疫治疗
近年来,以血管紧张素(angiotensin)及其特异性受体(AT1R)、β-肾上腺素能受体、α1-D肾上腺素受体等为靶点的肽类疫苗受到广泛关注[47,69-70]。动物实验表明,该类疫苗具有良好的免疫应答率,能降低大鼠及小鼠的血压,并可通过抑制基质金属蛋白酶和血管平滑肌细胞表型转变、减轻血管壁炎症和巨噬细胞浸润等非降压机制保护心血管、肾脏等靶器官[71-72]。此外,用于治疗肥胖和高脂血症的疫苗及巨噬细胞疫苗等也可能通过降压和心血管保护机制对部分AD患者的治疗和血压管理发挥积极作用[73-75],未来有望用于AD治疗。
4 小结
对于AD患者而言,仅通过服用降压药物很难达到理想的血压目标,且治疗中还会增加药物不良反应发生率并升高BPV,因此宜采用包括调整生活方式、手术、心理干预在内的综合管理措施以减少用药种类和剂量,改善患者的BPV和用药依从性,从而使得血压管理更加平稳有效。除经典的主动脉夹层腔内修复手术外,密网支架、带孔覆膜支架等不同类型移植物应用于主动脉分支区可进一步纠正AD的血流动力学异常,并为长期血压管理带来积极影响。此外,随着RSD理论、技术和器械的不断完善,其在AD后HT治疗中的作用将日益凸显。未来随着对新的降压靶点和疫苗研究的不断深入,AD群体血压管理将有更多治疗选择。
参考文献
[1]Whelton P K, Carey R M, Aronow W S, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines[J]. Hypertension, 2018, 71(6): e13-e115.
[2]Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people[J]. Lancet, 2014, 383(9932): 1899-1911.
[3]Eggebrecht H, Schmermund A, von Birgelen C, et al. Resistant hypertension in patients with chronic aortic disp[J]. J Hum Hypertens, 2005, 19(3): 227-231.
[4]Bossone E, Eagle K A. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes[J]. Nat Rev Cardiol, 2021, 18(5): 331-348.
[5]Thomas N. Hypertensive management[J]. Crit Care Nurs Clin North Am, 2023, 35(1): 31-38.
[6]Nienaber C A, Yuan X. Taming hypertension to prevent aortic disp: universal recognition of a “new normal” blood pressure?[J]. Circulation, 2022, 145(9): 645-647.
[7]Evangelista A, Isselbacher E M, Bossone E, et al. Insights from the international registry of acute aortic disp: a 20-year experience of collaborative clinical research[J]. Circulation, 2018, 137(17): 1846-1860.
[8]Shalaeva E V, Messerli F H. What is resistant arterial hypertension?[J]. Blood Press, 2023, 32(1): 2185457.
[9]Patel K V, Li X L, Kondamudi N, et al. Prevalence of apparent treatment-resistant hypertension in the United States according to the 2017 high blood pressure guideline[J]. Mayo Clin Proc, 2019, 94(5): 776-782.
[10]Januzzi J L, Sabatine M S, Choi J C, et al. Refractory systemic hypertension following type B aortic disp[J]. Am J Cardiol, 2001, 88(6): 686-688.
[11]Koracevic G, Lovic D, Zdravkovic M, et al. Long-lasting, resistant hypertension should be a part of the aortic disp risk score[J]. Hypertens Res, 2019, 42(11): 1836-1838.
[12]Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC)[J]. Eur Heart J, 2014, 35(41): 2873-2926.
[13]Li A, Mohetaer D, Zhao Q, et al. The relationship between renal artery involvement in Stanford B-type aortic disp and the short-term prognosis: a single-centre retrospective cohort study[J]. Heart Lung Circ, 2019, 28(8): 1261-1266.
[14]Ge Y P, Li C N, Li Y, et al. Relationship between renal function and renal artery involvement in acute Debakey type I aortic disp[J]. Heart Surg Forum, 2020, 23(4): E465-E469.
[15]van Bakel P A J, Henry M, Kim K M, et al. Imaging features of renal malperfusion in aortic disp[J]. Eur J Cardiothorac Surg, 2022, 61(4): 805-813.
[16]Lin Y J, Chen Y P, Peng Y C, et al. Symptoms of post-traumatic stress disor
der and associated risk factors in type A aortic disp[J]. J Cardiovasc Surg (Torino), 2021, 62(3): 286-293.
[17]Blakeslee-Carter J, Menon A J, Novak Z, et al. Association of mental health disorders and aortic disp[J]. Ann Vasc Surg, 2021, 77: 217-225.
[18]Camafort M, Ihm S H, Ruilope L M. Renal denervation for the treatment of hypertension and kidney disease[J]. Curr Opin Nephrol Hypertens, 2023, 32(6): 544-550.
[19]Tseilikman V, Komelkova M, Kondashevskaya M V, et al. A rat model of post-traumatic stress syndrome causes phenotype-associated morphological changes and hypofunction of the adrenal gland[J]. Int J Mol Sci, 2021, 22(24): 13235.
[20]Reed A B, Faizer R, James Valentine R. The impact of pre-existing blood pressure control in patients with acute aortic disps[J]. Vascular, 2022, 30(6): 1051-1057.
[21]Otaki Y, Watanabe T, Konta T, et al. Effect of hypertension on aortic artery disease-related mortality-3.8-year nationwide community-based prospective cohort study[J]. Circ J, 2018, 82(11): 2776-2782.
[22]Kimura N, Aizawa K, Kawahito K, et al. Outcomes of early-onset acute type a aortic disp-influence of etiologic factors[J]. Circ J, 2019, 83(2): 285-294.
[23]Gawinecka J, Schönrath F, Von Eckardstein A. Acute aortic disp: pathogenesis, risk factors and diagnosis[J]. Swiss Med Wkly, 2017, 147: w14489.
[24]Song C, Yu G Y, Feng X, et al. Impact of high blood pressure variability on the occurrence of acute type B aortic disp[J]. Vascular, 2020, 28(4): 413-420.
[25]Zhang L, Tian W, Feng R, et al. Prognostic impact of blood pressure variability on aortic disp patients after endovascular therapy[J]. Medicine (Baltimore), 2015, 94(38): e1591.
[26]Schutte A E, Kollias A, Stergiou G S. Blood pressure and its variability: classic and novel measurement techniques[J]. Nat Rev Cardiol, 2022, 19(10): 643-654.
[27]Jana S, Hu M, Shen M C, et al. Extracellular matrix, regional heterogeneity of the aorta, and aortic aneurysm[J]. Exp Mol Med, 2019, 51(12): 1-15.
[28]Lewis C E, Fine L J, Beddhu S, et al. Final report of a trial of intensive versus standard blood-pressure control[J]. N Engl J Med, 2021, 384(20): 1921-1930.
[29]Ribeiro F, Teixeira M, Alves A J, et al. Lifestyle medicine as a treatment for resistant hypertension[J]. Curr Hypertens Rep, 2023, 25(10): 313-328.
[30]Carey R M, Muntner P, Bosworth H B, et al. Prevention and control of hypertension: JACC health promotion series[J]. J Am Coll Cardiol, 2018, 72(11): 1278-1293.
[31]Xie H J, Li J C, Zhu X M, et al. Association between healthy lifestyle and the occurrence of cardiometabolic multimorbidity in hypertensive patients: a prospective cohort study of UK Biobank[J]. Cardiovasc Diabetol, 2022, 21(1): 199.
[32]Blumenthal J A, Hinderliter A L, Smith P J, et al. Effects of lifestyle modification on patients with resistant hypertension: results of the TRIUMPH randomized clinical trial[J]. Circulation, 2021, 144(15): 1212-1226.
[33]Flynn J T, Kaelber D C, Baker-Smith C M, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents[J]. Pediatrics, 2017, 140(3): e20171904.
[34]Yeghiazarians Y, Jneid H, Tietjens J R, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association[J]. Circulation, 2021, 144(3): e56-e67.
[35]Perger E, Pengo M F, Lombardi C. Hypertension and atrial fibrillation in obstructive sleep apnea: Is it a menopause issue?[J]. Maturitas, 2019, 124: 32-34.
[36]Akashiba T, Inoue Y, Uchimura N, et al. Sleep apnea syndrome (SAS) clinical practice guidelines 2020[J]. Respir Investig, 2022, 60(1): 3-32.
[37]Iida H, Kurita N, Takahashi S, et al. Salt intake and body weight correlate with higher blood pressure in the very elderly population: the Sukagawa study[J]. J Clin Hypertens (Greenwich), 2019, 21(7): 942-949.
[38]Huggins C E, Margerison C, Worsley A, et al. Influence of dietary modifications on the blood pressure response to antihypertensive medication[J]. Br J Nutr, 2011, 105(2): 248-255.
[39]Akhlaghi M. Dietary approaches to stop hypertension (DASH): potential mechanisms of action against risk factors of the metabolic syndrome[J]. Nutr Res Rev, 2020, 33(1): 1-18.
[40]Middlekauff H R, Cooper Z D, Strauss S B. Drugs of misuse: focus on vascular dysfunction[J]. Can J Cardiol, 2022, 38(9): 1364-1377.
[41]Isselbacher E M, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on clinical practice guidelines[J]. Circulation, 2022, 146(24): e334-e482.
[42]Chaddha A, Erickson S, Kline-Rogers E, et al. Medication adherence patterns in aortic disp survivors[J]. Indian J Med Res, 2018, 147(2): 183-188.
[43]Ishiyama Y, Hoshide S, Mizuno H, et al. Constipation-induced pressor effects as triggers for cardiovascular events[J]. J Clin Hypertens (Greenwich), 2019, 21(3): 421-425.
[44]Tehrani A Y, White Z, Milad N, et al. Blood pressure-independent inhibition of Marfan aortic root widening by the angiotensin Ⅱ receptor blocker valsartan[J]. Physiol Rep, 2021, 9(10): e14877.
[45]Hofmann Bowman M A, Eagle K A, Milewicz D M. Update on clinical trials of losartan with and without β-Blockers to block aneurysm growth in patients with Marfan syndrome: a review[J]. JAMA Cardiol, 2019, 4(7): 702-707.
[46]Strauss M H, Hall A S, Narkiewicz K. The combination of beta-blockers and ACE inhibitors across the spectrum of cardiovascular diseases[J]. Cardiovasc Drugs Ther, 2023, 37(4): 757-770.
[47]Gao Q N, Xu L, Cai J. New drug targets for hypertension: a literature review[J]. Biochim Biophys Acta Mol Basis Dis, 2021, 1867(3): 166037.
[48]Filippone E J, Naccarelli G V, Foy A J. Controversies in hypertension V: resistant and refractory hypertension[J]. Am J Med, 2024, 137(1): 12-22.
[49]Peacock E, Krousel-Wood M. Adherence to antihypertensive therapy[J]. Med Clin North Am, 2017, 101(1): 229-245.
[50]Tajeu G S, Kent S T, Kronish I M, et al. Trends in antihypertensive medication discontinuation and low adherence among Medicare beneficiaries initiating treatment from 2007 to 2012[J]. Hypertension, 2016, 68(3): 565-575.
[51]Gwadry-Sridhar F H, Manias E, Lal L, et al. Impact of interventions on medication adherence and blood pressure control in patients with essential hypertension: a systematic review by the ISPOR Medication Adherence and Persistence Special Interest Group[J]. Value Health, 2013, 16(5): 863-871.
[52]Martin G, Patel N, Grant Y, et al. Antihypertensive medication adherence in chronic type B aortic disp is an important consideration in the management debate[J]. J Vasc Surg, 2018, 68(3): 693-699.e2.
[53]Usai M V, Nugroho N T, Oberhuber A, et al. Influence of TEVAR on blood pressure in subacute type B aortic disp (TBAD) patients with refractory and non-refractory arterial hypertension[J]. Int Angiol, 2021, 40(1): 60-66.
[54]Deshpande A A, Pandey N N, Shaw M, et al. Evaluation of remodeling of visceral arteries and impact on renal function post-endovascular repair of type B aortic disp vis-a-vis baseline visceral artery morphology[J]. Vasc Endovascular Surg, 2022, 56(6): 553-560.
[55]Wang L C, Lin M H, Yu J J, et al. The impact of bariatric surgery versus non-surgical treatment on blood pressure: systematic review and meta-analysis[J]. Obes Surg, 2021, 31(11): 4970-4984.
[56]Sénéchal-Dumais I, Auclair A, Leclerc J, et al. Effect of bariatric surgery on blood pressure response to exercise in a severely obese population[J]. Blood Press Monit, 2021, 26(5): 357-363.
[57]Hawkins D N, Faler B J, Choi Y U, et al. Time course of blood pressure decrease after bariatric surgery in normoten-sive and hypertensive patients[J]. Obes Surg, 2018, 28(7): 1845-1851.
[58]Pang K P, Pang E B, Pang K A, et al. Upper airway surgery for obstructive sleep apnea reduces blood pressure[J]. Laryngoscope, 2018, 128(2): 523-527.
[59]Lim J H, Park P, Wee J H, et al. Evaluation of the success of obstructive sleep apnea surgery using criteria based on long-term symptoms and incident hypertension[J]. Eur Arch Otorhinolaryngol, 2018, 275(4): 1015-1022.
[60]Akinseye O A, Ralston W F, Johnson K C, et al. Renal sympathetic denervation: a comprehensive review[J]. Curr Probl Cardiol, 2021, 46(3): 100598.
[61]Doumas M, Faselis C, Papademetriou V. Renal sympathetic denervation and systemic hypertension[J]. Am J Cardiol, 2010, 105(4): 570-576.
[62]Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study[J]. Circulation, 2011, 123(18): 1940-1946.
[63]García-Touchard A, Maranillo E, Mompeo B, et al. Microdisp of the human renal nervous system: implications for performing renal denervation procedures[J]. Hypertension, 2020, 76(4): 1240-1246.
[64]Divchev D, Turan G, Rehders T, et al. Renal sympathetic denervation in patients with aortic disp[J]. J Interv Cardiol, 2014, 27(3): 334-339.
[65]Trimarchi S, Eagle K A, Nienaber C A, et al. Importance of refractory pain and hypertension in acute type B aortic disp: insights from the International Registry of Acute Aortic Disp (IRAD)[J]. Circulation, 2010, 122(13): 1283-1289.
[66]Ukena C, Mahfoud F, Kindermann I, et al. Cardiorespira-tory response to exercise after renal sympathetic denervation in patients with resistant hypertension[J]. J Am Coll Cardiol, 2011, 58(11): 1176-1182.
[67]Lenski D, Kindermann I, Lenski M, et al. Anxiety, depression, quality of life and stress in patients with resistant hypertension before and after catheter-based renal sympathetic denervation[J]. EuroIntervention, 2013, 9(6): 700-708.
[68]Warchol-Celinska E, Prejbisz A, Kadziela J, et al. Renal denervation in resistant hypertension and obstructive sleep apnea: randomized proof-of-concept phase Ⅱ trial[J]. Hypertension, 2018, 72(2): 381-390.
[69]Ke F, Kuang W L, Hu X J, et al. A novel vaccine targeting β1-adrenergic receptor[J]. Hypertens Res, 2023, 46(6): 1582-1595.
[70]Li C, Yan X L, Wu D Y, et al. Vaccine targeted alpha 1D-adrenergic receptor for hypertension[J]. Hypertension, 2019, 74(6): 1551-1562.
[71]Kurashiki T, Miyake T, Nakagami H, et al. Prevention of progression of aortic aneurysm by peptide vaccine against Ang Ⅱ(angiotensin Ⅱ) in a rat model[J]. Hypertension, 2020, 76(6): 1879-1888.
[72]Zhang H R, Liao M Y, Cao M S, et al. ATRQβ-001 vaccine prevents experimental abdominal aortic aneurysms[J]. J Am Heart Assoc, 2019, 8(18): e012341.
[73]Chyu K Y, Zhao X N, Zhou J C, et al. Immunization using ApoB-100 peptide-linked nanoparticles reduces atheroscler-osis[J]. JCI Insight, 2022, 7(11): e149741.
[74]Natsis M, Antza C, Doundoulakis I, et al. Hypertension in obesity: novel insights[J]. Curr Hypertens Rev, 2020, 16(1): 30-36.
[75]Wang F Z, Zhang Z, Fang A P, et al. Macrophage foam cell-targeting immunization attenuates atherosclerosis[J]. Front Immunol, 2018, 9: 3127.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言













#血压管理# #主动脉夹层#
141