方案与建议|中国精神疾病外科治疗专家共识
2024-11-01 中国神经精神疾病杂志 中国神经精神疾病杂志 发表于上海
本专家共识旨在为从事精神疾病外科治疗的临床工作人员制定外科治疗技术应用的专业标准,最大程度上提高治疗效果、促进该治疗技术未来的发展。
摘 要 《中国精神疾病外科治疗专家共识》由全国神经外科、精神科等相关领域专家编写。专家共识根据截至2023年12 月发表的临床研究并基于循证医学标准,发布神经外科手术在精神疾病临床中的治疗方案推荐,涵盖疾病包括: 强迫症、抑郁症、抽动症、双相障碍、神经性厌食症、物质使用相关障碍、精神分裂症等。本专家共识介绍了临床上神经外科手术治疗精神疾病的安全性和有效性,并初步规范了治疗流程和操作技术,旨在为从事精神疾病外科治疗的临床工作人员制定外科治疗技术应用的专业标准,最大程度上提高治疗效果、促进该治疗技术未来的发展。
关键词
神经外科学;精神病学;神经调控;专家共识;难治性精神疾病;强迫症;抑郁症;抽动症;双相障碍;神经性厌食症;物质使用相关障碍;精神分裂症
过去的数十年中,大多数精神障碍在中国人群中的患病率持续增加[1]。根据《国际疾病分类》(International Classification of Diseases,ICD)和《精神障碍诊断与统计手册》(Diagnostic and Statistical Manual of Mental Disorders,DSM)标准,精神障碍的终生患病率约为16.6%,在我国14亿人口基数现状下,这一患病率代表着数量庞大的患者群体与巨大的疾病负担[1]。大多数精神障碍可以通过药物、心理以及非侵入性的干预措施(如电休克治疗)进行有效治疗。然而,相当一部分患者在经历足量、足疗程的相关治疗后,依然存在改善欠佳、症状复发或者不可接受的治疗相关不良反应等问题[2-3],对于这类治疗效果不佳的精神障碍,可以在权衡之后考虑使用神经外科手术进行治疗,如立体定向毁损手术或脑深部电刺激(deep brain stimulation,DBS)等,这些神经外科治疗方法在药物难治性的强迫症(obsessive compulsive disorder, OCD)、重性抑郁障碍(major depressive disorder, MDD)、抽动症(tic disorders,TD)、药物滥用/成瘾(substance abuse/addiction)和神经性厌食症(anorexia nervosa,AN)等精神障碍中已有大量成功的临床报道,并且许多技术已成功地应用于临床实践[4]。在全世界范围内,接受手术的大部分难治性患者获得了持久、可观的症状缓解,并恢复了基本正常的生活。外科手术治疗对于多数系统性药物治疗无效的患者是必要的或是最后的选择[4-5]。
现代立体定向神经外科利用计算机计划系统将CT、MR图像融合,辅以标准的神经解剖图谱,实现可视的、个体化的脑区靶点定位,精度误差约0.1~0.3 mm,使多种精神障碍治疗效果较前有了明显提高、不良反应明显减少或减轻[5]。精神障碍外科治疗在近年取得了长足的进步[6],多种先进的神经影像及电生理技术可有效提升治疗效果。相关共识撰写年代较早[2-3, 5],临床证据不足,对其适应证、禁忌证及围术期疾病管理缺乏统一的规范,相关共识仍然需要不断发展和完善[2-3, 5- 6]。为了进一步规范我国精神障碍的外科手术治疗,提高诊疗水平,本共识由中国医师协会精神科医师分会精神疾病外科治疗联盟与中国医师协会神经调控专业委员会牵头,成立编写小组(名单见正文后),上海交通大学医学院附属瑞金医院孙伯民教授担任编写组长,来自全国相关领域专家根据我国临床研究过程中的关键方法学问题,对精神疾病外科治疗的临床诊疗指南、共识、综述类文献及高质量临床研究文章等证据进行检索与评价,基于2009及2010版指南,更新和修订了本共识[2-3]。
在共识伊始,本文声明外科干预应作为精神障碍传统治疗方法效果不满意或者无法耐受传统治疗方法患者的补充治疗手段,而不应作为确诊后患者的首选治疗方法。本共识分为两部分:第1部分包括精神疾病手术需要满足的通用标准与建议,第2部分在循证医学原则下,参考国内外指南、常用证据分级和推荐强度系统[7], 提出了对强迫症、抑郁症、抽动症、双相障碍、神经性厌食症、物质使用相关障碍、精神分裂症等疾病的适应证、禁忌证的临床推荐及手术方式建议。其他难治性精神疾病在进行充分的伦理、患者获益分析之后也可参考本共识进行治疗选择。
1 开展精神疾病外科治疗的通用标准与要求
1.1 开展精神疾病外科治疗的准入标准 ①三级甲等医院或具有资质的专科医院。②具有普通神经外科诊断、治疗经验及神经外科急救条件。③医院必须拥有MRI、CT等用于影像采集的设备。④拥有一支专门的精神外科治疗团队[包括精神科(或临床心理科)、神经外科、神经内科、神经影像、神经电生理、医学伦理学专家]负责包括患者的选择、术前评估、手术方案的选择、手术治疗、术后随访的全过程并能够提供手术后患者的后续治疗和关怀。团队中至少包括3名以上的精神科高级职称医师、2名以上的经过正规立体定向神经外科专科培训的高年资神经外科医师。⑤拥有可用于手术靶点定位的立体定向系统、射频毁损系统(如行核团毁损手术)、电生理监测系统。⑥手术治疗必须取得当地卫生行政管理部门批准的资质,经医院伦理委员会批准,并具有完善的患者知情与告知系统。
1.2 术前评估 (1) 术前一般评估:需完善神经外科手术术前一般状况评估,包括血常规、电解质、凝血功能、肝肾功能等术前常规检验,完善胸片、心电图等术前常规检查。完善头颅CT及MRI等立体定向神经外科手术术前必要检查,排除严重心脑器质性病变等手术禁忌证。
(2) 精神疾病的诊断及评估由精神科专家或多学科团队共同完成,另可根据具体病例选用以下通用评估量表[2]对精神状态、生活质量、认知功能等进行评估,包括:一般健康问卷(general health questionnaire,GHQ)、精神现状检查、神经精神病学评估表(schedules for clinical assessment in neuropsychiatry,SCAN)、DSM-Ⅳ结构性临床晤谈(structured clinical interview for DSM-Ⅳ disorders, SCID)、整体功能评估表(global assessment function,GAF)、生活质量晤谈(quality of life index,QOLI)、日常生活活动能力量表(activity of daily living,ADL)、蒙特利尔认知评估(Montreal cognitive assessment,MoCA),健康调查简表(the MOS item short from health survey,SF-36),希恩残疾量表(Sheehan disability scale,SDS)、社会功能缺陷筛选量表(social disability screening schedule,SDSS)以及社会支持评定量表(social support rate scale,SSRS)、明尼苏达多相人格调查表(Minnesota multiphasic personality inventory,MMPI)、国际人格障碍检查(international personality disorder examination,IPDE)等。
(3) 会诊:术前应经精神科与神经外科医师会诊。精神科医师的重点分析包括:精神疾病的诊断、鉴别诊断;是否为难治性疾病,以往的药物与心理行为治疗是否合理;适应证及靶症状与禁忌证;手术效果预估;家属与患者的期望、术后支持功能与监管情况;是否涉及法律与伦理问题等。神经外科医师则重点分析禁忌证、并发症、靶点选择等内容。
1.3 精神外科手术适应证与禁忌证 精神外科手术的目的是缓解患者精神疾病症状,恢复或改善精神功能,使患者适应社会工作和生活,提高患者及家属的生活质量。患者必须是经过有资质的、经验丰富的精神科专科医生正规充分治疗后未能奏效的难治性病例。术前必须告知患者和家属手术的必要性、安全性、可能带来的效益,以及手术可能产生的并发症及不可预测的风险。
以下列出精神疾病外科治疗的通用适应证与禁忌证,对于单个病种来说,除了满足以下列出标准之外,还应满足后续分病种单独列出的适应证与禁忌证。
1.3.1 适应证 ①符合进行一般颅脑外科手术的前提条件。②符合ICD-10/ICD-11或DSM-Ⅳ/DSM-5中对应精神障碍的诊断标准。③病程较长,经足量、足疗程的非手术治疗措施(通常包括心理、行为、药物、电休克治疗等,其中使用药物的数量、剂量和时间依照不同疾病具有不同的划分标准,见后分病种部分)治疗后仍属于难治性,或出现不能耐受的药物不良反应,导致无法继续使用药物治疗,或患者强烈拒绝进行除手术治疗外的其他治疗措施,如电休克等。④疾病给患者带来极大痛苦,严重影响工作和生活质量,具有极高的致残性。⑤患者及家属充分理解手术可能带来的效果、手术风险以及手术可能产生的不良反应及并发症,甚至可能带来的术前无法预测的后果,有些后果可能是严重的。⑥自知力完好的符合适应证的患者应在完全了解手术过程、接受手术疗效及相关并发症后签署知情同意书,无自知力或自知力受损的患者,应取得监护人知情同意后进行手术治疗。
1.3.2 禁忌证 ①存在平扫或增强MRI检查的禁忌证(除使用CT进行手术定位外);② 精神活性物质等引起的相关精神障碍(除2.6中所涉及的物质使用相关障碍);③严重的共患病,(a)严重的人格障碍,(b)器质性脑疾病、大脑结构异常,(c)严重的其他神经系统疾病(如癫痫等),(d)其他严重影响手术耐受或生存期的疾病;④ 相关手术靶点处受损或颅内存在无法手术的情况(术区脑梗死、动脉瘤、高颅压等);⑤妊娠。
1.4 手术方式的选择 目前主要手术方式包括射频毁损及DBS。常用的手术靶点包括内囊前肢(anterior limb of internal capsule,ALIC)、腹侧内囊/腹侧纹状体(ventral capsule/ventral striatum,VC/VS)、扣带回(cingulate)、伏隔核(nucleus accumbus,NAc)、杏仁核(amygdala,Amy)、内侧苍白球(globus pallidus internus,GPi)、丘脑底核(subthalamic nucleus,STN)等[8]。此外,在经过充分的伦理审核后,以磁共振引导的聚焦超声(magnetic resonance guided focused ultrasound,MRgFUS)为代表的新一代不开颅的神经调控治疗手术也逐渐增多[9-10]。目前的文献及经验还不能够明确治疗某种疾病的最佳手术方式,精神外科团队可根据患者的具体诊断、症状及需求来选择具体手术方式。一般不主张一次手术选择多个部位,第一次手术疗效不佳可在3~6个月后再次手术;如有患者病情复杂、单个手术部位具有解剖变异或结构异常等特殊情况,可酌情、谨慎考虑采用多部位手术。具体的手术靶点选择、证据及依据欧洲神经学会联盟提出的推荐级别[7]见本共识第2部分。
1.5 术后管理
1.5.1 药物管理及辅助治疗 一般在1年内不予改变患者术前的精神药物治疗;应在精神科医生指导下,遵循国内或国际相关指南或共识制定和调整药物及辅助治疗方案。
1.5.2 患者教育及术后护理 患者的教育建议在术前评估过程中尽早开始,内容包括:手术可能带来的实际收益和潜在的并发症、手术有难以解决或改善的临床症状甚至存在无效的风险、手术不能根治疾病和术后仍需坚持精神药物及心理治疗并在精神科和神经外科进行规律随访。
术后护理首先是安全护理,包括预防患者出现自伤自残等危及生命的行为,防止患者因情绪激动出现伤人或自伤行为,在无法控制时适当予以约束等。同时,在营养科、精神科及心理科医生的指导下予以患者合理营养、心理疏导以及社会活动参与等,提高患者生命质量。
1.5.3 DBS术后程控 开机:通常待患者的脑水肿好转、一般情况良好后即可开机,时间约为1~4周。开机前应复查CT或MRI明确电极位置。初始设置为单极刺激,通过单极刺激的筛选以识别触点急性刺激的不良和治疗效果,以确定慢性刺激使用的电极配置。通常情况下,DBS频率100~210 Hz,脉宽范围90~450 μs,电压2~8 V,刺激电流约2~15 mA。在每组参数设置下,刺激测试持续1~2 min,与无刺激期间交替进行。程控期间记录患者描述的任何不良效应,包括对感觉、运动功能、情绪或思维的影响。如果在某个刺激阈值出现不良反应,则维持此参数直至不良反应消失或维持1 min。
慢性刺激:与运动障碍的DBS治疗类似,刺激调整是一个迭代的过程,其基于程控医生对患者疗效和耐受性的判断。以 VC/VS刺激为例,当腹侧接触点激活并为负时,通常可以实现治疗效果和耐受性的最佳组合。患者需要在整个治疗过程中密切监测精神状态的恶化或与刺激相关的不良反应。
1.6 并发症管理
1.6.1 手术并发症 立体定向毁损手术短期并发症包括不同程度的头痛、发热、尿失禁、睡眠障碍以及疲惫等,以上症状一般在术后1周至1个月逐渐消失,不引起严重后果。长期并发症(包括人格改变、记忆丧失、嗜睡等)较少见(发生于<5%的患者),目前暂无较好的处理措施。
DBS手术并发症包括颅内出血、感染、术后癫痫发作、切口疼痛等。高龄、高血压、脑血管疾患、反复多次的微电极穿刺记录、穿刺针道与脑室过近被认为是发生出血的危险因素。手术路径建议尽量避免经过脑沟、脑室;术中严格无菌操作,尽量减少穿刺次数[11];术后应严密观察患者的血压及神经系统体征。颅内出血的处理原则类同其他病因的出血,可按照神经外科常规处理,若需要进行手术,术中应尽可能保留电极,减少移位,以便使DBS的预期作用仍可能实现[12]。
1.6.2 硬件并发症 对于DBS治疗的患者,可能发生的硬件并发症包括电极移位、感染、刺激器外露、电极或导线断裂等。感染、刺激器外露的患者在必要的清创和抗感染治疗下,如不能有效控制,则建议尽早去除颅内电极、延伸导线或脉冲发生器,待感染得到有效控制后,再行相应处理。
1.6.3 刺激相关并发症 对于DBS治疗的患者,开机后由于颅内电极刺激靶点及周围结构出现的不良反应称为刺激相关并发症。SCC-DBS最常见的可逆性刺激相关并发症为抑郁加重、焦虑加重、失眠加重;VC/VS-DBS最常见的可逆的刺激相关并发症为抑郁症状加重及轻躁狂状态;电极刺激伏隔核可能会导致相关不良反应的出现,如健忘、动机增加、行为异常、社交减退等[13-16]。刺激相关并发症大部分可通过调节DBS刺激参数减轻。这需要程控医生与患者及精神科医生结合触点位置共同探讨最适合的刺激参数及触点以达到最佳刺激效果且减少并发症发生。
2 分病种手术适应证、禁忌证及围术期管理
2.1 强迫症
2.1.1 适应证 ①年龄≥18岁;②发病>3年,且由于强迫症导致显著的功能损害,耶鲁-布朗强迫量表(Yale-Brown obsessive compulsive scale,Y-BOCS)症状评分处于“严重”范围(25分及以上)[17];③对于药物治疗方式存在抵抗[药物治疗需至少已使用2种选择性5-羟色胺再摄取抑制剂(SSRIs)或抗精神病药,其中一种需是氯米帕明,并且已达到FDA规定的最大耐受剂量;上述治疗时间至少达3个月];须接受至少12~20次认知行为疗法而无明显改善。
2.1.2 禁忌证 见“开展精神疾病外科治疗的通用标准与要求”。
2.1.3 术前评估 ①强迫症状评分:YBOCS;②相关症状评分:汉密尔顿抑郁评定量表(Hamilton depression rating scale,HAMD)、汉密尔顿焦虑评定量表(Hamilton anxiety rating scale,HAMA)等。
2.1.4 治疗推荐 见表1。
表1 强迫症外科治疗证据及推荐Tab.1 Evidences and recommendations of surgical treatment for OCD
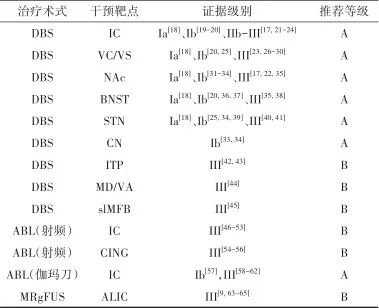
注:ABL, 毁损; IC,内囊; NAc, 伏隔核; BNST,终纹床核; CN, 尾状核; ITP,丘脑下脚; MD/VA, 丘脑背内侧核/丘脑腹前核; slMFB,内侧前脑束上外侧支; CING, 扣带回。
2.2 抑郁症
2.2.1 适应证 ①年龄≥18岁;②病程超过2年;③重度抑郁,HAMD≥17分或蒙哥马利和艾森贝格抑郁症等级量表(Montgomery-Asberg depression rating scale,MADRS)≥22分[66-68] ;或具有非常严重的、无法控制的自杀行为;④对至少3种剂量和疗程适当的抗抑郁药物(3种药物至少有2种化学结构是不同的)治疗反应不良[69]。
2.2.2 禁忌证 见 “开展精神疾病外科治疗的通用标准与要求”。
2.2.3 术前评估 ①抑郁症状评分:他评量表包括HAMD、MADRS等;自评量表包括贝克抑郁量表(Beck depression inventory,BDI)、抑郁和躯体症状量表(depression and somatic symptoms scale,DSSS)、抑郁症症状快速自评量表(quick inventory of depressive symptomatology-self-report,QIDS-SR16)等。②焦虑症状评分:HAMA、贝克焦虑量表(Beck anxiety inventory,BAI)等。
2.2.4 治疗推荐 见表2。
表2 抑郁症外科治疗证据及推荐Tab.2 Evidences and recommendations of surgical treatment for depression
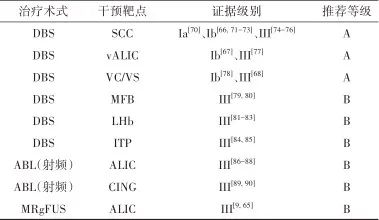
注: SCC, 胼胝体下扣带回; vALIC, 腹侧内囊前肢; MFB,内侧前脑束; LHB, 外侧缰核; ITP, 丘脑下脚; CING,扣带回。
2.3 抽动症
2.3.1 适应证 ①严重的抽动症状,耶鲁大体抽动严重程度量表(Yale global tic severity scale,YGTSS)评分≥35分[91-92];②精神症状严重、对日常功能有显著影响;③在至少3种不同类别药物治疗和至少6个月认知行为干预治疗下无显著改善。
2.3.2 禁忌证 见“开展精神疾病外科治疗的通用标准与要求”。
2.3.3 术前评估 ①抽动症状评分:YGTSS。②伴随精神症状评分:注意力缺陷多动障碍测试量表(attention-deficit/hyperactivity disorder rating scale IV,ADHD-RS-Ⅳ)、YBOCS、HAMD、BDI、HAMA及BAI等。
2.3.4 治疗推荐 见表3。
表3 抽动症外科治疗证据及推荐Tab.3 Evidences and recommendations of surgical treatment for Tic disorders
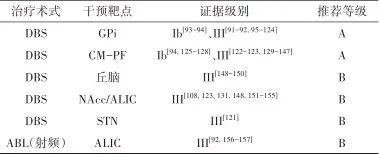
注: CM-Pf, 中央中核束-旁核复合体; NAcc,伏隔核。
2.4 双相障碍
2.4.1 适应证 ①年龄≥18岁;②病程超过2年;③重度抑郁,HAMD≥20分[76],或具有非常严重的、无法控制的自杀行为;④对至少3种剂量和疗程适当的抗抑郁药物(3种药物至少有2种化学结构是不同的)治疗反应不良的难治性抑郁[69]。
2.4.2 禁忌证 见“开展精神疾病外科治疗的通用标准与要求”。
2.4.3 术前评估 双相障碍可分为双相I型障碍、双相II型障碍、环性心境障碍、物质/药物所致的双相障碍、由于其他躯体疾病所致的双相障碍、其他特定的双相障碍和未特定的双相障碍。双相障碍可伴发多种精神障碍,包括焦虑障碍(最常见)、进食障碍、物质使用障碍等,区分患者的主要诊断与次要诊断需要经验丰富的精神科医生对于患者病程充分了解后进行判断。
(1)抑郁评定量表:他评量表(HAMD、MADRS等);自评量表(BDI、DSSS、抑郁症症状快速自评量表等)。
(2)躁狂评定量表:杨氏躁狂评定量表(Young manic rating scale,YMRS)。
(3)焦虑评定量表: HAMA、BAI等。
2.4.4 治疗推荐 见表4。
表4 双相障碍外科治疗证据及推荐Tab.4 Evidences and recommendations of surgical treatment for bipolar disorders

注: Hb,缰核。
2.5 神经性厌食症
2.5.1 适应证 ①病程≥3年,且病程中持续存在功能损害;② 对于药物治疗与心理治疗的联合治疗方式存在抵抗(药物治疗需至少已使用2种抗抑郁药或抗精神病药,并且足量足疗程;心理治疗需由精神科或精神病院具有心理治疗经验的心理医师规范化治疗);③患者目前的生理(极度严重的营养不良)或精神状态(既往存在自杀尝试)处于生命危急的情形。
2.5.2 禁忌证 见“开展精神疾病外科治疗的通用标准与要求”。
2.5.3 术前评估 (1)一般情况除通用指南部分的一般状况评估以外,AN还需评估:(a)躯体高风险评估[快速评估躯体风险的指标包括体质量指数(body mass index,BMI)、血压、心率、肌力] ,BMI<14 kg/m2、血压低于80 mmHg/50 mmHg、心率<40次/min、体质量每周下降超1 kg、卧位坐起或蹲起时需辅助等均可被视作躯体高风险的指征;(b)再喂养风险评估,治疗初期需高度警惕再喂养综合征(refeeding syndrome,RFS)的发生。RFS是指机体经过长期饥饿或营养不良,重新摄入营养物质导致以低磷血症为特征的电解质代谢紊乱及由此产生的心率失常、急性心力衰竭、休克、谵妄等一系列症状,具有潜在致命危险。由于AN患者普遍存在严重的营养不良及其所导致的极低BMI,电解质紊乱,凝血功能及心血管功能低下、内分泌系统紊乱等诸多躯体症状,需要特别注意AN患者的围术期管理。
(2)精神疾病相关评估量表:(a)AN的精神病理评估,目前可在临床应用的评估工具包括进食障碍检查自评问卷第6版(the questionnaire version of the eating disorders examination,EDEQ-6.0)、进食态度自评问卷(eating attitudes test,EAT-26)和进食障碍调查量表第2版(Eating disorder inventory,EDI-2);(b)精神疾病共病,除了进食障碍相关量表的评估以外,还需要对AN患者进行精神疾病共病、认知功能以及社会功能评定。评估工具包括HAMD、 HAMA、BDI、BAI、YBOCS。同时,还需认真询问病史,结合量表判断是否伴发抑郁症、焦虑障碍、强迫症、双相障碍和酒精或物质滥用。
2.5.4 术后管理 (1)营养支持营养治疗(包括饮食监管及禁止暴食和呕吐行为)是AN最重要、最紧急、最基本的治疗,也是实现体质量增加、预防AN死亡的必要干预措施[160]。营养治疗的目标是充分恢复正常体质量、恢复正常的饮食习惯、纠正营养不良导致的多种生理问题。当患者胃肠道功能恢复后应尽早进行营养治疗。由于目前缺乏AN患者立体定向毁损手术或DBS术后营养支持管理的研究,可综合一般神经外科手术术后营养管理以及中国和国际AN营养治疗相关指南[160-161]。一般遵循经口进食、起始少量、逐渐增加的原则,肠外营养仅用于严重病例抢救生命的短期治疗方法,必要时请营养科会诊。临床上,体质量指数即使恢复到参考范围的底限(成年人BMI 18.5 kg/m2),患者的生理功能仍然可能无法恢复,因此,应由营养专家根据患者营养不良的严重程度,提供不同级别的营养重建方案。由于AN的疾病特征使患者对营养治疗的依从性差,在进餐时可能出现较大情绪波动,甚至与家属或医护人员出现冲突,因此营养治疗必须与其他治疗方式联合进行。目前临床上对于显著低体质量的个体,营养重建至少要经历3个阶段——稳定化阶段、恢复阶段、巩固维持阶段,具体可参阅《中国神经性厌食症诊疗专家共识》。需要注意再喂养综合症,尤其是术后患者进食欲望增强后,更容易引发相关风险。
(2)药物管理。由于DBS和立体定向毁损手术的作用机制与药物不同,一般不改变患者术前的精神药物治疗。应在精神科医生指导下,遵循《中国神经性厌食症诊疗专家共识》以及国际相关指南及推荐系统制定和调整药物方案[160-161]。
(3)心理治疗。一般不调整患者术前的心理治疗方案,建议患者继续遵循术前心理治疗方案。对于青少年AN患者,国内及国外的进食障碍诊治指南均将基于家庭的治疗(family-based treatment,FBT)列为首选[160, 162-163]。但对于成人AN患者尚无证据表明某一种治疗方式优于其他治疗方式。
(4) DBS程控见“术后药物及程控管理”。
(5) 并发症的预防及处理:由于AN患者常合并营养不良,患者易出现术后低钠血症、低钾血症以及低磷血症等电解质紊乱。因此,应在围术期严密监测患者电解质情况,及时予以补充及调整。术后癫痫发生可能部分地归因于低钠血症[164]。
2.5.5 治疗推荐 见表5。
表5 神经性厌食症外科治疗证据及推荐Tab.5 Evidences and recommendations of surgical treatment for anorexia nervosa
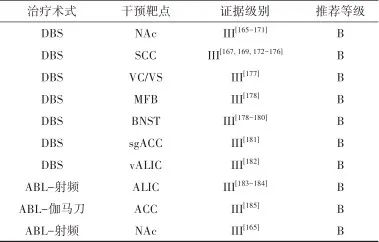
注: BNST, 终纹床核; sgACC,膝下前扣带回; ACC, 前扣带回。
2.6 物质使用相关障碍
2.6.1 适应证 (1) 对于酒精相关障碍的患者,在症状上应满足同时存在ICD-10或DSM-5规定的6项以上临床症状,或存在明显的酒精中毒或酒精戒断症状,或伴发其他难治性精神疾病;在病程上应满足症状持续时间至少12个月;非手术治疗无效,包括心理、行为、药物、物理治疗等, 其中使用至少3种以上的治疗方案。
(2) 对于毒品相关障碍的患者,包括阿片类物质相关障碍、致幻剂相关障碍、兴奋剂相关障碍、大麻相关障碍,在症状上应满足同时存在ICD-10或DSM-5规定的6项以上临床症状,或存在毒品相关的犯罪史,或存在阿片类物质、甲基苯丙胺类物质、大麻类物质中毒史,或存在毒品滥用诱发的其他难治性精神疾病;药物治疗无法控制的复吸。
(3) 对于烟草相关障碍的患者,在症状上应满足同时存在ICD-10或DSM-5规定的6项以上临床症状,而且同时存在其他难治性精神疾病。
(4) 非物质相关成瘾障碍目前不建议使用精神外科治疗。
2.6.2 禁忌证 见“开展精神疾病外科治疗的通用标准与要求”。
2.6.3 术前评估 除了常规检查之外,物质使用相关障碍的术前评估也应包括对症状严重程度的评估、对耐受程度的评估、对在受控制环境下的缓解情况的评估、对戒断相关的行为和躯体症状的评估、对犯罪风险与自杀风险的评估、对多种精神活性物质共同使用情况的评估、对物质使用导致中毒的评估、对躯体疾病共病的评估、对其他精神疾病共病的评估[186]。
2.6.4 术后药物管理 术后管理主要涉及术后药物管理与术后成瘾物质管理。对于术后药物管理,出于安全性考虑,手术前后应保持原有的治疗药物不变,在术后在精神内科医生的指导下进行药物剂量的调整,如病情稳定可分阶段逐步减小剂量直至停药。
如果出现了戒断相关的急性不良反应,则应对症处理。对于术后成瘾物质管理,如患者为酒精成瘾或烟草成瘾,不应直接停止相关物质的使用以避免不良反应的发生;如患者为毒品成瘾,应在戒断毒品的同时采取替代治疗,例如美沙酮、丁丙诺啡等[186]。
2.6.5 术后程控 (1) 开机:通常待患者的脑水肿好转、一般情况良好后即可开机,时间约为1~4周。开机前可复查MRI或CT以明确电极的位置。刺激参数根据患者的反应来调整。对于严重的毒品成瘾,可酌情早期开机以控制症状。对于初始开机参数来说,频率选择130~180 Hz,脉宽选择90~210 μs,电压选择3.0~5.5 V。
(2) 后期调控:物质使用相关障碍的脑深部电刺激治疗可在开机后14 d内达到最佳效果。对于难治性毒品成瘾,术后6个月是复吸的高危时间窗口[14]。因此在这一时间窗口内应进行细致的刺激参数调整以达到最佳治疗效果、避免复吸现象发生。由于电刺激核团产生的其他不良反应也应当在后期调控中避免。
2.6.6 不良反应 戒断与撤退相关并发症:突发的精神活性物质戒断可能具有严重后果,包括躯体体征和症状(自主神经相关的生命体征变化、腹痛、头痛、恶心、呕吐)、谵妄、强烈的渴求、焦虑、抑郁、激越、惊厥、癫痫发作、精神病性症状、认知功能损害及其他精神活性物质的替代性使用等[186]。
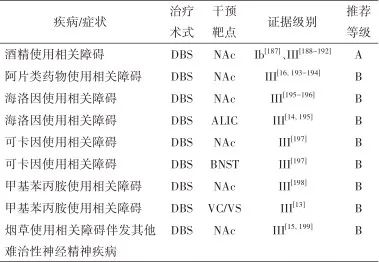
2.6.7 治疗推荐 见表6。
表6 成瘾相关疾病外科治疗证据及推荐Tab.6 Evidences and recommendations of surgical treatment for addiction
2.7 精神分裂症
2.7.1 适应证 ①年龄≥18岁;②病程≥3年,阳性症状持续2年以上无明显缓解;③阳性和阴性症状量表(positive and negative symptoms scale,PANSS)“妄想”、“幻觉”、“猜疑/被害”、“不寻常思维”项中至少两项超过4分或有一项超过6分[或简明精神病评定量表(brief psychiatric rating scale,BPRS)≥35分],临床疗效总评量表( clinical global impression,CGI)≥4分[200-201];④对至少3种抗精神病药物(3种药物至少有2种化学结构是不同的,须包括氯氮平)在适当的剂量和疗程治疗后仍改善不佳。
2.7.2 禁忌证 见“开展精神疾病外科治疗的通用标准与要求”。
2.7.3 术前评估 (1)阳性和阴性症状评定量表:PANSS、BPRS、谵妄分级量表(delirium rating scale,DRS)等。
(2)整体评定量表:CGI等。
2.7.4 治疗推荐 见表7。
表7 精神分裂症外科治疗证据及推荐Tab.7 Evidences and recommendations of surgical treatment for schizophrenia
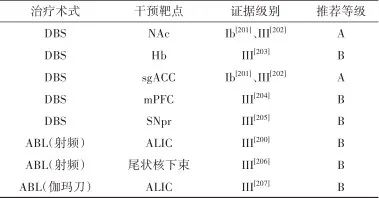
注: mPFC, 内侧前额叶; SNpr,黒质网状部。
参考文献:
1. HUANG Y, WANG Y, WANG H, et al. Prevalence of mental disorders in China: a cross-pal epidemiological study[J]. Lancet Psychiatry, 2019, 6(3): 211-224.
2. 中华医学会神经外科分会, 中国医师协会神经外科分会. 精神外科临床指南(建议稿)[J]. 中国神经精神疾病杂志, 2009, 35(8): 449.
3. 杨理荣, 任廷文, 李瑞惠, 等. 精神外科治疗指南(建议稿)[J]. 立体定向和功能性神经外科杂志, 2010, 23(6): 361-366.
4. NUTTIN B, WU H, MAYBERG H, et al. Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders[J]. J Neurol Neurosurg Psychiatry, 2014, 85(9): 1003-1008.
5. 杨理荣, 任廷文, 李瑞惠, 等. 现代精神外科适应证与禁忌证[J]. 中国民康医学, 2011, 23(21): 2693-3696.
6. 张陈诚, 李殿友, 占世坤, 等. "立体定向神经外科技术治疗精神疾病专家共识"解读[J]. 中华神经医学杂志, 2015, 14(2): 109-111.
7. BRAININ M, BARNES M, BARON J C, et al. Guidance for the preparation of neurological management guidelines by EFNS scientific task forces--revised recommendations 2004[J]. Eur J Neurol, 2004, 11(9): 577-581.
8. NEUMAIER F, PATERNO M, ALPDOGAN S, et al. Surgical Approaches in Psychiatry: A Survey of the World Literature on Psychosurgery[J]. World Neurosurg, 2017, 97: 603.
9. DAVIDSON B, HAMANI C, RABIN J S, et al. Magnetic resonance-guided focused ultrasound capsulotomy for refractory obsessive compulsive disorder and major depressive disorder: clinical and imaging results from two phase I trials[J]. Mol Psychiatry, 2020, 25(9): 1946-1957.
10. DOEBROESSY M D, RAMANATHAN C, ASHOURI VAJARI D, et al. Neuromodulation in Psychiatric disorders: Experimental and Clinical evidence for reward and motivation network Deep Brain Stimulation: Focus on the medial forebrain bundle[J]. Eur J Neurosci, 2021, 53(1): 89-113.
11. ZRINZO L, FOLTYNIE T, LIMOUSIN P, et al. Reducing hemorrhagic complications in functional neurosurgery: a large case series and systematic literature review[J]. J Neurosurg, 2012, 116(1): 84-94.
12. WANG X, WANG J, ZHAO H, et al. Clinical analysis and treatment of symptomatic intracranial hemorrhage after deep brain stimulation surgery[J]. Br J Neurosurg, 2017, 31(2): 217-222.
13. GE S, CHEN Y, LI N, et al. Deep Brain Stimulation of Nucleus Accumbens for Methamphetamine Addiction: Two Case Reports[J]. World Neurosurg, 2019, 122: 512-517.
14. VALENCIA-ALFONSO C E, LUIGJES J, SMOLDERS R, et al. Effective Deep Brain Stimulation in Heroin Addiction: A Case Report with Complementary Intracranial Electroencephalogram[J]. Biol Psychiatry, 2012, 71(8): E35-E7.
15. MANTIONE M, VAN DE BRINK W, SCHUURMAN P R, et al. Smoking Cessation and Weight Loss After Chronic Deep Brain Stimulation of the Nucleus Accumbens: Therapeutic and Research Implications: Case Report[J]. Neurosurgery, 2010, 66(1): 218.
16. KUHN J, MÖLLER M, TREPPMANN J F, et al. Deep brain stimulation of the nucleus accumbens and its usefulness in severe opioid addiction[J]. Molecular Psychiatry, 2014, 19(2): 145-146.
17. XIONG B, LI B, WEN R, et al. Use of differential stimulation of the nucleus accumbens and anterior limb of the internal capsule to improve outcomes of obsessive-compulsive disorder[J]. J Neurosurg, 2023: 139(5): 1376-1385.
18. GADOT R, NAJERA R, HIRANI S, et al. Efficacy of deep brain stimulation for treatment-resistant obsessive-compulsive disorder: systematic review and meta-analysis[J]. J Neurol Neurosurg Psychiatry, 2022: jnnp-2021-328738.
19. ABELSON J L, CURTIS G C, SAGHER O, et al. Deep brain stimulation for refractory obsessive-compulsive disorder[J]. Biol Psychiatry, 2005, 57(5): 510-516.
20. GREENBERG B D, GABRIELS L A, MALONE D A, JR., et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience[J]. Mol Psychiatry, 2010, 15(1): 64-79.
21. OOMS P, BLANKERS M, FIGEE M, et al. Cost-effectiveness of deep brain stimulation versus treatment as usual for obsessive-compulsive disorderJ]. Brain Stimul, 2017, 10(4): 836-842.
22. BESZLEJ J A, SIWICKI D, FILA-WITECKA K, et al. Deep brain stimulation in obsessive-compulsive disorder - case report of two patients[J]. Psychiatria Polska, 2019, 53(4): 807-824.
23. GUPTA A, KHANNA S, JAIN R. Deep brain stimulation of ventral internal capsule for refractory obsessive-compulsive disorder[J]. Indian J Psychiatry, 2019, 61(5): 532-536.
24. DENYS D, GRAAT I, MOCKING R, et al. Efficacy of Deep Brain Stimulation of the Ventral Anterior Limb of the Internal Capsule for Refractory Obsessive-Compulsive Disorder: A Clinical Cohort of 70 Patients[J]. Am J Psychiatry, 2020, 177(3): 265-271.
25. TYAGI H, APERGIS-SCHOUTE A M, AKRAM H, et al. A Randomized Trial Directly Comparing Ventral Capsule and Anteromedial Subthalamic Nucleus Stimulation in Obsessive-Compulsive Disorder: Clinical and Imaging Evidence for Dissociable Effects [J]. Biol Psychiatry, 2019, 85(9): 726-734.
26. GREENBERG B D, MALONE D A, FRIEHS G M, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder [J]. Neuropsychopharmacology, 2006, 31(11): 2384-2393.
27. ROH D, CHANG W S, CHANG J W, et al. Long-term follow-up of deep brain stimulation for refractory obsessive-compulsive disorder [J]. Psychiatry Res, 2012, 200(2-3): 1067-1070.
28. TSAI H C, CHANG C H, PAN J I, et al. Acute stimulation effect of the ventral capsule/ventral striatum in patients with refractory obsessive-compulsive disorder - a double-blinded trial [J]. Neuropsychiatr Dis Treat, 2014, 10: 63-69.
29. HOLLAND M T, TRAPP N T, MCCORMICK L M, et al. Deep Brain Stimulation for Obsessive-Compulsive Disorder: A Long Term Naturalistic Follow Up Study in a Single Institution[J]. Front Psychiatry, 2020, 11:55.
30. MENCHÓN J M, REAL E, ALONSO P, et al. A prospective international multi-center study on safety and efficacy of deep brain stimulation for resistant obsessive-compulsive disorder[J]. Mol Psychiatry, 2021, 26(4): 1234-1247.
31. DENYS D, MANTIONE M, FIGEE M, et al. Deep Brain Stimulation of the Nucleus Accumbens for Treatment-Refractory Obsessive-Compulsive Disorder[J]. Arch Gen Psychiatry, 2010, 67(10): 1061-1068.
32. HUFF W, LENARTZ D, SCHORMANN M, et al. Unilateral deep brain stimulation of the nucleus accumbens in patients with treatment-resistant obsessive-compulsive disorder: Outcomes after one year[J]. Clin Neurol Neurosurg, 2010, 112(2): 137-143.
33. BARCIA J A, AVECILLAS-CHASÍN J M, NOMBELA C, et al. Personalized striatal targets for deep brain stimulation in obsessive-compulsive disorder[J]. Brain Stimul, 2019, 12(3): 724-734.
34. WELTER M L, ALVES DOS SANTOS J F, CLAIR A H, et al. Deep Brain Stimulation of the Subthalamic, Accumbens, or Caudate Nuclei for Patients With Severe Obsessive-Compulsive Disorder: A Randomized Crossover Controlled Study[J]. Biol Psychiatry, 2021, 90(10): e45-e47.
35. ISLAM L, FRANZINI A, MESSINA G, et al. Deep Brain Stimulation of the Nucleus Accumbens and Bed Nucleus of Stria Terminalis for Obsessive-Compulsive Disorder: A Case Series[J]. World Neurosurg, 2015, 83(4): 657-663.
36. LUYTEN L, HENDRICKX S, RAYMAEKERS S, et al. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder[J]. Mol Psychiatry, 2016, 21(9): 1272-1280.
37. MOSLEY P E, WINDELS F, MORRIS J, et al. A randomised, double-blind, sham-controlled trial of deep brain stimulation of the bed nucleus of the stria terminalis for treatment-resistant obsessive-compulsive disorder[J]. Transl Psychiatry, 2021, 11(1): 190.
38. FARRAND S, EVANS A H, MANGELSDORF S, et al. Deep brain stimulation for severe treatment-resistant obsessive-compulsive disorder: An open-label case series[J]. Aust N Z J Psychiatry, 2018, 52(7): 699-708.
39. MALLET L, POLOSAN M, JAAFARI N, et al. Subthalamic Nucleus Stimulation in Severe Obsessive-Compulsive Disorder[J]. N Engl J Med, 2008, 359(20): 2121-2134.
40. VOON V, DROUX F, MORRIS L, et al. Decisional impulsivity and the associative-limbic subthalamic nucleus in obsessive-compulsive disorder: stimulation and connectivity[J]. Brain, 2017, 140(2): 442-56.
41. CHABARDES S, KRACK P, PIALLAT B, et al. Deep brain stimulation of the subthalamic nucleus in obsessive-compulsives disorders: long-term follow-up of an open, prospective, observational cohort[J]. J Neurol Neurosur Ps, 2020, 91(12): 1349-56.
42. JIMÉNEZ F, NICOLINI H, LOZANO A M, et al. Electrical Stimulation of the Inferior Thalamic Peduncle in the Treatment of Major Depression and Obsessive Compulsive Disorders [J]. World Neurosurg, 2013, 80(3-4): S30, e17-e25.
43. LEE D J, DALLAPIAZZA R F, DE VLOO P, et al. Inferior thalamic peduncle deep brain stimulation for treatment-refractory obsessive-compulsive disorder: A phase 1 pilot trial[J]. Brain Stimul, 2019, 12(2): 344-352.
44. MAAROUF M, NEUDORFER C, EL MAJDOUB F, et al. Deep Brain Stimulation of Medial Dorsal and Ventral Anterior Nucleus of the Thalamus in OCD: A Retrospective Case Series[J]. PloS one, 2016, 11(8):e0160750.
45. COENEN V A, SCHLAEPFER T E, GOLL P, et al. The medial forebrain bundle as a target for deep brain stimulation for obsessive-compulsive disorder[J]. Cns Spectrums, 2017, 22(3): 282-289.
46. OLIVER B, GASCON J, APARICIO A, et al. Bilateral anterior capsulotomy for refractory obsessive-compulsive disorders[J]. Stereotact Funct Neurosurg, 2003, 81(1-4): 90-95.
47. LIU K, ZHANG H, LIU C, et al. Stereotactic treatment of refractory obsessive compulsive disorder by bilateral capsulotomy with 3 years follow-up[J]. J Clin Neurosci, 2008, 15(6): 622-629.
48. CSIGO K, HARSANYI A, DEMETER G, et al. Long-term follow-up of patients with obsessive-compulsive disorder treated by anterior capsulotomy: a neuropsychological study[J]. J Affect Disord, 2010, 126(1-2): 198-205.
49. ZHANG Q J, WANG W H, WEI X P. Long-term efficacy of stereotactic bilateral anterior cingulotomy and bilateral anterior capsulotomy as a treatment for refractory obsessive-compulsive disorder[J]. Stereotact Funct Neurosurg, 2013, 91(4): 258-261.
50. ZHAN S, LIU W, LI D, et al. Long-term follow-up of bilateral anterior capsulotomy in patients with refractory obsessive-compulsive disorder[J]. Clin Neurol Neurosurg, 2014, 119: 91-95.
51. GONG F, LI P, LI B, et al. A study of cognitive function in treatment-refractory obsessive-compulsive disorder treated with capsulotomy[J]. J Neurosurg, 2018, 128(2): 583-595.
52. YIN D, ZHANG C, LV Q, et al. Dissociable Frontostriatal Connectivity: Mechanism and Predictor of the Clinical Efficacy of Capsulotomy in Obsessive-Compulsive Disorder[J]. Biol Psychiatry, 2018, 84(12): 926-936.
53. LIU H B, ZHONG Q, WANG W. Bilateral anterior capsulotomy for patients with refractory obsessive-compulsive disorder: A multicenter, long-term, follow-up study[J]. Neurol India, 2017, 65(4): 770-776.
54. JUNG H H, KIM C H, CHANG J H, et al. Bilateral anterior cingulotomy for refractory obsessive-compulsive disorder: Long-term follow-up results[J]. Stereotact Funct Neurosurg, 2006, 84(4): 184-189.
55. SHETH S A, NEAL J, TANGHERLINI F, et al. Limbic system surgery for treatment-refractory obsessive-compulsive disorder: a prospective long-term follow-up of 64 patients[J]. J Neurosurg, 2013, 118(3): 491-497.
56. YANG J C, GINAT D T, DOUGHERTY D D, et al. Lesion analysis for cingulotomy and limbic leucotomy: comparison and correlation with clinical outcomes[J]. J Neurosurg, 2014, 120(1): 152-163.
57. LOPES A C, GREENBERG B D, CANTERAS M M, et al. Gamma ventral capsulotomy for obsessive-compulsive disorder: a randomized clinical trial[J]. JAMA psychiatry, 2014, 71(9): 1066-1076.
58. KONDZIOLKA D, FLICKINGER J C, HUDAK R. Results following gamma knife radiosurgical anterior capsulotomies for obsessive compulsive disorder[J]. Neurosurgery, 2011, 68(1): 28-32; discussion 23-23.
59. RASMUSSEN S A, NOREN G, GREENBERG B D, et al. Gamma Ventral Capsulotomy in Intractable Obsessive-Compulsive DisorderJ]. Biol Psychiatry, 2018, 84(5): 355-364.
60. RUCK C, KARLSSON A, STEELE J D, et al. Capsulotomy for obsessive-compulsive disorder: long-term follow-up of 25 patients[J]. Arch Gen Psychiatry, 2008, 65(8): 914-921.
61. SHEEHAN J P, PATTERSON G, SCHLESINGER D, et al. gamma knife surgery anterior capsulotomy for severe and refractory obsessive-compulsive disorder[J]. J Neurosurg, 2013, 119(5): 1112-1118.
62. SPATOLA G, MARTINEZ-ALVAREZ R, MARTINEZ-MORENO N, et al. Results of Gamma Knife anterior capsulotomy for refractory obsessive-compulsive disorder: results in a series of 10 consecutive patients[J]. J Neurosurg, 2018,131(2): 376-383.
63. JUNG H H, KIM S J, ROH D, et al. Bilateral thermal capsulotomy with MR-guided focused ultrasound for patients with treatment-refractory obsessive-compulsive disorder: a proof-of-concept study[J]. Mol Psychiatry, 2015, 20(10): 1205-1211.
64. KIM S J, ROH D, JUNG H H, et al. A study of novel bilateral thermal capsulotomy with focused ultrasound for treatment-refractory obsessive-compulsive disorder: 2-year follow-up[J]. J Psychiatry Neurosci, 2018, 43(5): 327-337.
65. DAVIDSON B, HAMANI C, HUANG Y, et al. Magnetic Resonance-Guided Focused Ultrasound Capsulotomy for Treatment-Resistant Psychiatric Disorders[J]. Oper Neurosurg, 2020, 19(6): 741-749.
66. HOLTZHEIMER P E, HUSAIN M M, LISANBY S H, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial[J]. Lancet Psychiatry, 2017, 4(11): 839-849.
67. BERGFELD I O, MANTIONE M, HOOGENDOORN M L, et al. Deep Brain Stimulation of the Ventral Anterior Limb of the Internal Capsule for Treatment-Resistant Depression: A Randomized Clinical Trial[J]. JAMA Psychiatry, 2016, 73(5): 456-464.
68. LAI Y, DAI L, WANG T, et al. Structural and functional correlates of the response to deep brain stimulation at ventral capsule/ventral striatum region for treatment-resistant depression[J]. J Neurol Neurosurg Psychiatry, 2023, 94(5): 379-388.
69. RUSH A J, TRIVEDI M H, WISNIEWSKI S R, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report[J]. Am J Psychiatry, 2006, 163(11): 1905-1017.
70. SOBSTYL M, KUPRYJANIUK A, PROKOPIENKO M, et al. Subcallosal Cingulate Cortex Deep Brain Stimulation for Treatment-Resistant Depression: A Systematic Review[J]. Front Neurol, 2022, 13: 780481.
71. PUIGDEMONT D, PORTELLA M, PEREZ-EGEA R, et al. A randomized double-blind crossover trial of deep brain stimulation of the subcallosal cingulate gyrus in patients with treatment-resistant depression: a pilot study of relapse prevention[J]. J Psychiatry Neurosci, 2015, 40(4): 224-231.
72. RAMASUBBU R, CLARK D L, GOLDING S, et al. Long versus short pulse width subcallosal cingulate stimulation for treatment-resistant depression: a randomised, double-blind, crossover trial[J]. Lancet Psychiatry, 2020, 7(1): 29-40.
73. ALAGAPAN S, CHOI K S, HEISIG S, et al. Cingulate dynamics track depression recovery with deep brain stimulation[J]. Nature, 2023, 622(7981): 130-138.
74. PUIGDEMONT D, PÉREZ-EGEA R, PORTELLA M J, et al. Deep brain stimulation of the subcallosal cingulate gyrus: further evidence in treatment-resistant major depression[J]. Int J Neuropsychopharmacol, 2012, 15(1): 121-133.
75. CROWELL A L, RIVA-POSSE P, HOLTZHEIMER P E, et al. Long-Term Outcomes of Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Depression[J]. Am J Psychiatry, 2019, 176(11): 949-956.
76. HOLTZHEIMER P E, KELLEY M E, GROSS R E, et al. Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Unipolar and Bipolar Depression[J]. Arch Gen Psychiatry, 2012, 69(2): 150-158.
77. VAN DER WAL J M, BERGFELD I O, LOK A, et al. Long-term deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression[J]. J Neurol Neurosurg Psychiatry, 2020, 91(2): 189-195.
78. DOUGHERTY D D, REZAI A R, CARPENTER L L, et al. A Randomized Sham-Controlled Trial of Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Chronic Treatment-Resistant Depression[J]. Biol Psychiatry, 2015, 78(4): 240-248.
79. FENOY A J, SCHULZ P, SELVARAJ S, et al. Deep brain stimulation of the medial forebrain bundle: Distinctive responses in resistant depression[J]. J Affect Disord, 2016, 203: 143-151.
80. FENOY A J, SCHULZ P E, SELVARAJ S, et al. A longitudinal study on deep brain stimulation of the medial forebrain bundle for treatment-resistant depression[J]. Transl Psychiatry, 2018, 8(1): 111.
81. SARTORIUS A, KIENING K L, KIRSCH P, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient[J]. Biol Psychiatry, 2010, 67(2): e9-e11.
82. KIENING K, SARTORIUS A. A new translational target for deep brain stimulation to treat depression[J]. EMBO Mol Med, 2013, 5(8): 1151-1153.
83. ZHANG C, ZHANG Y, LUO H, et al. Bilateral Habenula deep brain stimulation for treatment-resistant depression: clinical findings and electrophysiological features [J]. Transl Psychiatry, 2022, 12(1): 52.
84. KOPELL B H, GREENBERG B D. Anatomy and physiology of the basal ganglia: implications for DBS in psychiatry [J]. Neurosci Biobehav Rev, 2008, 32(3): 408-422.
85. JIMÉNEZ F, VELASCO F, SALIN-PASCUAL R, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle[J]. Neurosurgery, 2005, 57(3): 585-593; discussion -93.
86. AVECILLAS-CHASIN J M, HURWITZ T A, BOGOD N M, et al. An Analysis of Clinical Outcome and Tractography following Bilateral Anterior Capsulotomy for Depression[J]. Stereotact Funct Neurosurg, 2019, 97(5-6): 369-380.
87. CHRISTMAS D, ELJAMEL M S, BUTLER S, et al. Long term outcome of thermal anterior capsulotomy for chronic, treatment refractory depression[J]. J Neurol Neurosurg Psychiatry, 2011, 82(6): 594-600.
88. SUBRAMANIAN L, BRACHT T, JENKINS P, et al. Clinical improvements following bilateral anterior capsulotomy in treatment-resistant depression[J]. Psychol Med, 2017, 47(6): 1097-1106.
89. SHIELDS D C, ASAAD W, ESKANDAR E N, et al. Prospective assessment of stereotactic ablative surgery for intractable major depression[J]. Biol Psychiatry, 2008, 64(6): 449-454.
90. STEELE J D, CHRISTMAS D, ELJAMEL M S, et al. Anterior cingulotomy for major depression: Clinical outcome and relationship to lesion characteristics[J]. Biol Psychiatry, 2008, 63(7): 670-677.
91. KEFALOPOULOU Z, ZRINZO L, JAHANSHAHI M, et al. Bilateral globus pallidus stimulation for severe Tourette’s syndrome: a double-blind, randomised crossover trial[J]. Lancet Neurol, 2015, 14(6): 595-605.
92. ZHANG C, DENG Z, PAN Y, et al. Pallidal deep brain stimulation combined with capsulotomy for Tourette’s syndrome with psychiatric comorbidity[J]. J Neurosurg, 2019, 131(6): 1788-1796.
93. WELTER M L, HOUETO J L, THOBOIS S, et al. Anterior pallidal deep brain stimulation for Tourette’s syndrome: a randomised, double-blind, controlled trial[J]. Lancet Neurology, 2017, 16(8): 610-619.
94. MÜLLER-VAHL K R, SZEJKO N, SARYYEVA A, et al. Randomized double-blind sham-controlled trial of thalamic versus GPi stimulation in patients with severe medically refractory Gilles de la Tourette syndrome[J]. Brain Stimul, 2021, 14(3): 662-675.
95. DIEDERICH N J, KALTEIS K, STAMENKOVIC M, et al. Efficient internal pallidal stimulation in Gilles de la Tourette syndrome: a case report[J]. Mov Disord, 2005, 20(11): 1496-1499.
96. SHAHED J, POYSKY J, KENNEY C, et al. GPi deep brain stimulation for Tourette syndrome improves tics and psychiatric comorbidities[J]. Neurology, 2007, 68(2): 159-160.
97. DEHNING S, MEHRKENS J H, MÜLLER N, et al. Therapy-refractory Tourette syndrome: Beneficial outcome with globus pallidus internus deep brain stimulation[J]. Mov Disord, 2008, 23(9): 1300-1302.
98. MARTÍNEZ-FERNÁNDEZ R, ZRINZO L, AVILES-OLMOS I, et al. Deep Brain Stimulation for Gilles de la Tourette Syndrome: A Case Series Targeting Subregions of the Globus Pallidus Internus[J]. Mov Disord, 2011, 26(10): 1922-1930.
99. DONG S, ZHUANG P, ZHANG X H, et al. Unilateral Deep Brain Stimulation of the Right Globus Pallidus Internus in Patients with burette’s Syndrome: Two Cases with Outcomes After 1 Year and a Brief Review of the Literature[J]. J Int Med Res, 2012, 40(5): 2021-2028.
100. MASSANO J, SOUSA C, FOLTYNIE T, et al. Successful pallidal deep brain stimulation in 15-year-old with Tourette syndrome: 2-year follow-up[J]. J Neurol, 2013, 260(9): 2417-2419.
101. MOTLAGH M G, SMITH M E, LANDEROS-WEISENBERGER A, et al. Lessons Learned from Open-label Deep Brain Stimulation for Tourette Syndrome: Eight Cases over 7 Years[J]. Tremor Other Hyperkinet Mov, 2013, 3:tre-03-170-4428-1.
102. DEHNING S, LEITNER B, SCHENNACH R, et al. Functional outcome and quality of life in Tourette’s syndrome after deep brain stimulation of the posteroventrolateral globus pallidus internus: long-term follow-up [J]. World J Biol Psychiatry, 2014, 15(1): 66-75.
103. DONG S, ZHANG X H, LI J Y, et al. The benefits of low-frequency pallidal deep brain stimulation in a patient with Tourette syndrome[J]. Parkinsonism Relat Disord, 2014, 20(12): 1438-1439.
104. HUASEN B, MCCREARY R, EVANS J, et al. Cervical myelopathy secondary to Tourette’s syndrome managed by urgent deep brain stimulation[J]. Mov Disord, 2014, 29(4): 452-453.
105. NAIR G, EVANS A, BEAR R E, et al. The anteromedial GPi as a new target for deep brain stimulation in obsessive compulsive disorder[J]. J Clin Neurosci, 2014, 21(5): 815-821.
106. PATEL N, JIMENEZ-SHAHED J. Simultaneous improvement of tics and parkinsonism after pallidal DBS[J]. Parkinsonism Relat Disord, 2014, 20(9): 1022-1023.
107. SACHDEV P S, MOHAN A, CANNON E, et al. Deep Brain Stimulation of the Antero-Medial Globus Pallidus Interna for Tourette Syndrome[J]. PloS one, 2014, 9(8):e104926.
108. CANNON E, SILBURN P, COYNE T, et al. Deep Brain Stimulation of Anteromedial Globus Pallidus Interna for Severe Tourette’s Syndrome[J]. Am J Psychiatry, 2012, 169(8): 860-866.
109. ZHANG J G, GE Y, STEAD M, et al. Long-term Outcome of Globus Pallidus Internus Deep Brain Stimulation in Patients With Tourette Syndrome[J]. Mayo Clin Proc, 2014, 89(11): 1506-1514.
110. MORREALE F, KEFALOPOULOU Z, ZRINZO L, et al. Inhibitory Control on a Stop Signal Task in Tourette Syndrome before and after Deep Brain Stimulation of the Internal Segment of the Globus Pallidus[J]. Brain Sci, 2021, 11(4):461.
111. WÅRDELL K, KEFALOPOULOU Z, DICZFALUSY E, et al. Deep Brain Stimulation of the Pallidum Internum for Gilles de la Tourette Syndrome: A Patient-Specific Model-Based Simulation Study of the Electric Field[J]. Neuromodulation, 2015, 18(2): 90-96.
112. SMEETS A, DUITS A A, PLANTINGA B R, et al. Deep Brain Stimulation of the internal globus pallidus in refractory Tourette Syndrome[J]. Clin Neurol Neurosurg, 2016, 142: 54-59.
113. ZHANG X H, LI J Y, ZHANG Y Q, et al. Deep Brain Stimulation of the Globus Pallidus Internus in Patients with Intractable Tourette Syndrome: A 1-year Follow-up Study[J]. Chin Med J, 2016, 129(9): 1022-1027.
114. AKBARIAN-TEFAGHI L, AKRAM H, JOHANSSON J, et al. Refining the Deep Brain Stimulation Target within the Limbic Globus Pallidus Internus for Tourette Syndrome[J]. Stereotact Funct Neurosurg, 2017, 95(4): 251-258.
115. DWARAKANATH S, HEGDE A, KETAN J, et al. “I swear, I can’t stop it!” - A case of severe Tourette’s syndrome treated with deep brain stimulation of anteromedial globus pallidus interna[J]. Neurol India, 2017, 65(1): 99-102.
116. PICILLO M, ROHANI M, LOZANO A M, et al. Two indications, one target: Concomitant epilepsy and Tourettism treated with Centromedian/parafascicular thalamic stimulation[J]. Brain Stimul, 2017, 10(3): 711-713.
117. AZIMI A, PARVARESH M, SHAHIDI G, et al. Anteromedial GPi deep brain stimulation in Tourette syndrome: The first case series from Iran[J]. Clin Neurol Neurosurg, 2018, 172: 116-119.
118. DOSHI P K, RAMDASI R, THORVE S. Deep brain stimulation of anteromedial globus pallidus internus for severe Tourette syndrome[J]. Indian J Psychiatry, 2018, 60(1): 138-140.
119. ROSSI M, CERQUETTI D, CAMMAROTA A, et al. Tourette syndrome: Clinical benefit with unilateral stimulation after bilateral pallidal implant[J]. Mov Disord, 2019, 34(4): 580-582.
120. ZHANG C C, LI H X, PAN Y X, et al. Pallidal Neurostimulation and Capsulotomy for Malignant Tourette’s Syndrome[J]. Mov Disord Clinical Practice, 2019, 6(5): 393-395.
121. ZHU G Y, GENG X Y, ZHANG R L, et al. Deep brain stimulation modulates pallidal and subthalamic neural oscillations in Tourette’s syndrome[J]. Brain Behav, 2019, 9(12):e01450.
122. SERVELLO D, ZEKAJ E, SALEH C, et al. Deep Brain Stimulation in Gilles de la Tourette Syndrome: What Does the Future Hold? A Cohort of 48 Patients[J]. Neurosurgery, 2016, 78(1): 91-100.
123. SERVELLO D, PORTA M, SASSI M, et al. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation[J]. J Neurol Neurosurg Psychiatry, 2008, 79(2): 136-142.
124. SUN F Q, ZHANG X H, DONG S, et al. Effectiveness of Low-Frequency Pallidal Deep Brain Stimulation at 65 Hz in Tourette Syndrome[J]. Neuromodulation, 2022, 25(2): 286-295.
125. MACIUNAS R J, MADDUX B N, RILEY D E, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome[J]. J Neurosurg, 2007, 107(5): 1004-1014.
126. ACKERMANS L, DUITS A, van der LINDEN C, et al. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome[J]. Brain, 2011, 134: 832-844.
127. OKUN M S, FOOTE K D, WU S S, et al. A Trial of Scheduled Deep Brain Stimulation for Tourette Syndrome <i>Moving Away From Continuous Deep Brain Stimulation Paradigms[J]. JAMA Neurol, 2013, 70(1): 85-94.
128. BALDERMANN J C, KUHN J, SCHÜLLER T, et al. Thalamic deep brain stimulation for Tourette Syndrome: A naturalistic trial with brief randomized, double-blinded sham-controlled periods[J]. Brain Stimul, 2021, 14(5): 1059-1067.
129. BAJWA R J, DE LOTBINIÈRE A J, KING R A, et al. Deep brain stimulation in Tourette’s syndrome[J]. Mov Disord, 2007, 22(9): 1346-1350.
130. SERVELLO D, SASSI M, BRAMBILLA A, et al. Long-Term, Post-Deep Brain Stimulation Management of a Series of 36 Patients Affected With Refractory Gilles de la Tourette Syndrome[J]. Neuromodulation, 2010, 13(3): 187-194.
131. SERVELLO D, SASSI M, BRAMBILLA A, et al. De novo and rescue DBS leads for refractory Tourette syndrome patients with severe comorbid OCD: a multiple case report[J]. J Neurol, 2009, 256(9): 1533-1539.
132. MARCEGLIA S, SERVELLO D, FOFFANI G, et al. Thalamic Single-Unit and Local Field Potential Activity in Tourette Syndrome[J]. Mov Disord, 2010, 25(3): 300-308.
133. PULLEN S J, WALL C A, LEE K H, et al. Neuropsychiatric Outcome of an Adolescent Who Received Deep Brain Stimulation for Tourette’s Syndrome[J]. Case Rep Neurol Med, 2011, 2011: 209467.
134. KAIDO T, OTSUKI T, KANEKO Y, et al. Deep Brain Stimulation for Tourette Syndrome: A Prospective Pilot Study in Japan[J]. Neuromodulation, 2011, 14(2): 123-128.
135. LEE M W Y, AU-YEUNG M M, HUNG K N, et al. Deep brain stimulation in a Chinese Tourette’s syndrome patient [J]. Hong Kong Med J, 2011, 17(2): 147-150.
136. RZESNITZEK L, WÄCHTER T, KRÜGER R, et al. SUPPRESSION OF EXTRAPYRAMIDAL SIDE EFFECTS OF DOXEPIN BY THALAMIC DEEP BRAIN STIMULATION FOR TOURETTE SYNDROME[J]. Neurology, 2011, 77(18): 1708-1709.
137. SAVICA R, STEAD M, MACK K J, et al. Deep Brain Stimulation in Tourette Syndrome: A Description of 3 Patients With Excellent Outcome[J]. Mayo Clin Proc, 2012, 87(1): 59-62.
138. DUITS A, ACKERMANS L, CATH D, et al. Unfavourable outcome of deep brain stimulation in a Tourette patient with severe comorbidity[J]. Eur Child Adolesc Psychiatry, 2012, 21(9): 529-531.
139. POURFAR M H, BUDMAN C L, MOGILNER A Y. A Case of Deep Brain Stimulation for Tourette’s Complicated by Twiddler’s Syndrome[J]. Mov Disord clinical practice, 2015, 2(2): 192-193.
140. CURY R G, LOPEZ W O C, GHILARDI M G D, et al. Parallel improvement in anxiety and tics after DBS for medically intractable Tourette syndrome: A long-term follow-up[J]. Clin Neurol Neurosurg, 2016, 144: 33-35.
141. TESTINI P, ZHAO C Z, STEAD M, et al. Centromedian-Parafascicular Complex Deep Brain Stimulation for Tourette Syndrome: A Retrospective Study[J]. Mayo Clin Proc, 2016, 91(2): 218-225.
142. DOWD R S, POURFAR M, MOGILNER A Y. Deep brain stimulation for Tourette syndrome: a single-center series [J]. J Neurosurg, 2018, 128(2): 596-604.
143. KANO Y, MATSUDA N, NONAKA M, et al. Sensory phenomena and obsessive-compulsive symptoms in Tourette syndrome following deep brain stimulation: Two case reports[J]. J Clin Neurosci, 2018, 56: 199-201.
144. BRITO M, TEIXEIRA M J, MENDES M M, et al. Exploring the clinical outcomes after deep brain stimulation in Tourette syndrome[J]. J Neurol Sci, 2019, 402: 48-51.
145. ANDRADE P, HEIDEN P, HOEVELS M, et al. Modulation of Fibers to Motor Cortex during Thalamic DBS in Tourette Patients Correlates with Tic Reduction[J]. Brain Sci, 2020, 10(5):302.
146. HEIDEN P, HOEVELS M, BAYRAM D, et al. Connectivity Patterns of Deep Brain Stimulation Targets in Patients with Gilles de la Tourette Syndrome[J]. Brain Sci, 2021, 11(1):87.
147. KIMURA Y, IIJIMA K, TAKAYAMA Y, et al. Deep Brain Stimulation for Refractory Tourette Syndrome: Electrode Position and Clinical Outcome[J]. Neurol Med Chir, 2021, 61(1): 33-39.
148. KUHN J, BARTSCH C, LENARTZ D, et al. Clinical effectiveness of unilateral deep brain stimulation in Tourette syndrome[J]. Transl Psychiatry, 2011, 1(11):52.
149. HUYS D, BARTSCH C, KOESTER P, et al. Motor Improvement and Emotional Stabilization in Patients With Tourette Syndrome After Deep Brain Stimulation of the Ventral Anterior and Ventrolateral Motor Part of the Thalamus [J]. Biol Psychiatry, 2016, 79(5): 392-401.
150. RICHIERI R, BLACKMAN G, MUSIL R, et al. Positive clinical effects of gamma knife capsulotomy in a patient with deep brain stimulation-refractory Tourette Syndrome and Obsessive Compulsive Disorder[J]. Clin Neurol Neurosurg, 2018, 170: 34-37.
151. KUHN J, LENARTZ D, MAI J K, et al. Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndrome[J]. J Neurol, 2007, 254(7): 963-965.
152. NEUNER I, PODOLL K, LENARTZ D, et al. Deep brain stimulation in the nucleus accumbens for intractable Tourette’s syndrome: follow-up report of 36 months[J]. Biol Psychiatry, 2009, 65(4): e5-6.
153. BURDICK A, FOOTE K D, GOODMAN W, et al. Lack of benefit of accumbens/capsular deep brain stimulation in a patient with both tics and obsessive-compulsive disorder[J]. Neurocase, 2010, 16(4): 321-330.
154. SACHDEV P S, CANNON E, COYNE T J, et al. Bilateral deep brain stimulation of the nucleus accumbens for comorbid obsessive compulsive disorder and Tourette’s syndrome[J]. BMJ Case Rep, 2012, 2012:bcr2012006579.
155. DUARTE-BATISTA P, COELHO M, QUINTAS S, et al. Anterior Limb of Internal Capsule and Bed Nucleus of Stria Terminalis Stimulation for Gilles de la Tourette Syndrome with Obsessive-Compulsive Disorder in Adolescence: A Case of Success[J]. Stereotact Funct Neurosurg, 2020, 98(2): 95-103.
156. SUN B, KRAHL S E, ZHAN S, et al. Improved capsulotomy for refractory Tourette’s syndrome[J]. Stereotact Funct Neurosurg, 2005, 83(2-3): 55-56.
157. WANG S, FAN S, GAN Y, et al. Efficacy and safety of combined deep brain stimulation with capsulotomy for comorbid motor and psychiatric symptoms in Tourette’s syndrome: Experience and evidence[J]. Asian J Psychiatr, 2024, 94: 103960.
158. TORRES C V, EZQUIAGA E, NAVAS M, et al. Deep brain stimulation of the subcallosal cingulate for medication-resistant type I bipolar depression: case report[J]. Bipolar Disord, 2013, 15(6): 719-721.
159. ZHANG C, KIM S G, LI D, et al. Habenula deep brain stimulation for refractory bipolar disorder[J]. Brain Stimul, 2019, 12(5): 1298-300.
160. 陈涵, 陈妍, 韩慧琴, 等. 中国神经性厌食症诊疗专家共识 [J]. 中国全科医学, 2024, 27(5): 509-520.
161. MARIKAR D, REYNOLDS S, MOGHRABY O S. Junior MARSIPAN (Management of Really Sick Patients with Anorexia Nervosa)[J]. Arch Dis Child Educ Pract Ed, 2016, 101(3): 140-143.
162. LOCK J, LA VIA M C. Practice parameter for the assessment and treatment of children and adolescents with eating disorders[J]. J Am Acad Child Adolesc Psychiatry, 2015, 54(5): 412-425.
163. Scottish Intercollegiate Guidelines Network(SIGN). Eating disorders 2022 [EB/OL]. [2023-07-15]http: //www.sign.ac.uk.
164. LIPSMAN N, LAM E, VOLPINI M, et al. Deep brain stimulation of the subcallosal cingulate for treatment-refractory anorexia nervosa: 1 year follow-up of an open-label trial[J]. Lancet Psychiatry, 2017, 4(4): 285-294.
165. WANG J, CHANG C, GENG N, et al. Treatment of Intractable Anorexia Nervosa with Inactivation of the Nucleus Accumbens Using Stereotactic Surgery[J]. Stereotact Funct Neurosurg, 2013, 91(6): 364-372.
166. ZHANG H-W, LI D-Y, ZHAO J, et al. Metabolic Imaging of Deep Brain Stimulation in Anorexia Nervosa: A 18F-FDG PET/CT Study[J]. Clin Nucl Med, 2013, 38(12): 943-948.
167. VILLALBA MARTÍNEZ G, JUSTICIA A, SALGADO P, et al. A Randomized Trial of Deep Brain Stimulation to the Subcallosal Cingulate and Nucleus Accumbens in Patients with Treatment-Refractory, Chronic, and Severe Anorexia Nervosa: Initial Results at 6 Months of Follow Up[J]. J Clin Med, 2020, 9(6): 1946.
168. LIU W, ZHAN S, LI D, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory anorexia nervosa: A long-term follow-up study[J]. Brain Stimul, 2020, 13(3): 643-649.
169. PÉREZ V, VILLALBA-MARTÍNEZ G, ELICES M, et al. Cognitive and quality-of-life related factors of body mass index (BMI) improvement after deep brain stimulation in the subcallosal cingulate and nucleus accumbens in treatment-refractory chronic anorexia nervosa[J]. Eur Eat Disord Rev, 2022, 30(4): 353-363.
170. SCAIFE J C, ERAIFEJ J, GREEN A L, et al. Deep Brain Stimulation of the Nucleus Accumbens in Severe Enduring Anorexia Nervosa: A Pilot Study[J]. Front Behav Neurosci, 2022, 16:842184.
171. ARROTEIA I F, HUSCH A, BANIASADI M, et al. Impressive weight gain after deep brain stimulation of nucleus accumbens in treatment-resistant bulimic anorexia nervosa[J]. BMJ Case Rep, 2020, 13(11): e239316.
172. LIPSMAN N, WOODSIDE D B, GIACOBBE P, et al. Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial[J]. Lancet, 2013, 381(9875): 1361-1370.
173. HAYES D J, LIPSMAN N, CHEN D Q, et al. Subcallosal Cingulate Connectivity in Anorexia Nervosa Patients Differs From Healthy Controls: A Multi-tensor Tractography Study[J]. Brain Stimul, 2015, 8(4): 758-768.
174. LIPSMAN N, LAM E, VOLPINI M, et al. Deep brain stimulation of the subcallosal cingulate for treatment-refractory anorexia nervosa: 1 year follow-up of an open-label trial[J]. Lancet Psychiatry, 2017, 4(4): 285-294.
175. de VLOO P, LAM E, ELIAS G J, et al. Long-term follow-up of deep brain stimulation for anorexia nervosa [J]. J Neurol Neurosurg Psychiatry, 2021, 92(10): 1135-1136.
176. VLOO P D, LAM E, ELIAS G J, et al. Long-term follow-up of deep brain stimulation for anorexia nervosa [J]. J Neurol Neurosurg Psychiatry, 2021, 92(10): 1135-1136.
177. MCLAUGHLIN N C, DIDIE E R, MACHADO A G, et al. Improvements in anorexia symptoms after deep brain stimulation for intractable obsessive-compulsive disorder[J]. Biol Psychiatry, 2013, 73(9): e29-e31.
178. BLOMSTEDT P, NAESSTRÖM M, BODLUND O. Deep brain stimulation in the bed nucleus of the stria terminalis and medial forebrain bundle in a patient with major depressive disorder and anorexia nervosa[J]. Clin Case Rep, 2017, 5(5): 679-684.
179. MANUELLI M, FRANZINI A, GALENTINO R, et al. Changes in eating behavior after deep brain stimulation for anorexia nervosa. A case study[J]. Eat Weight Disord 2020, 25(5): 1481-1486.
180. VISMARA M E M, FRANZINI A, MANUELLI M, et al. P.406 Deep brain stimulation in treating anorexia nervosa and comorbid obsessive compulsive disorder: a 12 months follow-up case study[J]. Eur Neuropsychopharm, 2020, 40: S232-S233.
181. ISRAËL M, STEIGER H, KOLIVAKIS T, et al. Deep Brain Stimulation in the Subgenual Cingulate Cortex for an Intractable Eating Disorder[J]. Biol Psychiatry, 2010, 67(9): e53-e54.
182. OUDIJN M S, MOCKING R J T, WIJNKER R R, et al. Deep brain stimulation of the ventral anterior limb of the capsula interna in patients with treatment-refractory anorexia nervosa[J]. Brain Stimul, 2021, 14(6): 1528-1530.
183. LIU W, LI D, SUN F, et al. Long-Term Follow-up Study of MRI-Guided Bilateral Anterior Capsulotomy in Patients With Refractory Anorexia Nervosa[J]. Neurosurgery, 2018, 83(1): 86-92.
184. WANG J, CHANG C, GENG N, et al. Treatment of intractable anorexia nervosa with inactivation of the nucleus accumbens using stereotactic surgery[J]. Stereotact Funct Neurosurg, 2013, 91(6): 364-372.
185. MARTÍNEZ-ÁLVAREZ R. Radiosurgery for Behavioral Disorders[J]. Prog Neurol Surg, 2019, 34: 289-297.
186. AMERICAN PSYCHIATRIC ASSOCIATION D, ASSOCIATION A P. Diagnostic and statistical manual of mental disorders: DSM-5[M]. Washingtom: American psychiatric association Washington, DC, 2013.
187. BACH P, LUDERER M, MÜLLER U J, et al. Deep brain stimulation of the nucleus accumbens in treatment-resistant alcohol use disorder: a double-blind randomized controlled multi-center trial[J]. Transl Psychiatry, 2023, 13(1):49.
188. MÜLLER U J, STURM V, VOGES J, et al. Nucleus Accumbens Deep Brain Stimulation for Alcohol Addiction - Safety and Clinical Long-term Results of a Pilot Trial[J]. Pharmacopsychiatry, 2016, 49(4): 170-173.
189. DAVIDSON B, GIACOBBE P, GEORGE T P, et al. Deep brain stimulation of the nucleus accumbens in the treatment of severe alcohol use disorder: a phase I pilot trial[J]. Mol Psychiatry, 2022, 27(10): 3992-4000.
190. HELDMANN M, BERDING G, VOGES J, et al. Deep Brain Stimulation of Nucleus Accumbens Region in Alcoholism Affects Reward Processing[J]. PLoS One, 2012, 7(5)::e36572.
191. KUHN J, LENARTZ D, HUFF W, et al. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? [J]. J Neurol Neurosurg Psychiatry, 2007, 78(10): 1152-1153.
192. KUHN J, GRÜNDLER T O J, BAUER R, et al. Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring[J]. Addict Biol, 2011, 16(4): 620-623.
193. MAHONEY J J, HAUT M W, HODDER S L, et al. Deep Brain Stimulation of the Nucleus Accumbens/Ventral Capsule for Severe and Intractable Opioid and Benzodiazepine Use Disorder[J]. Exp Clin Psychopharmacol, 2021, 29(2): 210-215.
194. REZAI A R, MAHONEY J J, III, RANJAN M, et al. Safety and feasibility clinical trial of nucleus accumbens deep brain stimulation for treatment-refractory opioid use disorder[J]. J Neurosurg, 2024, 140(1): 231-239.
195. CHEN L, LI N, GE S, et al. Long-term results after deep brain stimulation of nucleus accumbens and the anterior limb of the internal capsule for preventing heroin relapse: An open-label pilot study[J]. Brain Stimul, 2019, 12(1): 175-183.
196. ZHOU H Y, XU J W, JIANG J Y. Deep Brain Stimulation of Nucleus Accumbens on Heroin-Seeking Behaviors: A Case Report[J]. Biol Psychiatry, 2011, 69(11): E41-E2.
197. GONÇALVES-FERREIRA A, DO COUTO F S, CAMPOS A R, et al. Deep Brain Stimulation for Refractory Cocaine Dependence[J]. Biol Psychiatry, 2016, 79(11): E87-E9.
198. ZHANG C, WEI H, ZHANG Y, et al. Increased dopamine transporter levels following nucleus accumbens deep brain stimulation in methamphetamine use disorder: A case report[J]. Brain Stimul, 2019, 12(4): 1055-1057.
199. KUHN J, BAUER R, POHL S, et al. Observations on Unaided Smoking Cessation after Deep Brain Stimulation of the Nucleus Accumbens[J]. Eur Addict Res, 2009, 15(4): 196-201.
200. LIU W, HAO Q, ZHAN S, et al. Long-term follow-up of mri-guided bilateral anterior capsulotomy in patients with refractory schizophrenia[J]. Stereotact Funct Neurosurg, 2014, 92(3): 145-152.
201. CORRIPIO I, ROLDÁN A, SARRÓ S, et al. Deep brain stimulation in treatment resistant schizophrenia: A pilot randomized cross-over clinical trial[J]. EBioMedicine, 2020, 51: 102568.
202. BIOQUE M, RUMIA J, ROLDAN P, et al. Deep brain stimulation and digital monitoring for patients with treatment-resistant schizophrenia and bipolar disorder: A case series[J]. Rev Psiquiatr Salud Ment, 2023:S1888-9891(23)00013-7.
203. WANG Y, ZHANG C, ZHANG Y, et al. Habenula deep brain stimulation for intractable schizophrenia: a pilot study[J]. Neurosurg focus, 2020, 49(1):E9.
204. CORRIPIO I, SARRÓ S, MCKENNA P J, et al. Clinical Improvement in a Treatment-Resistant Patient With Schizophrenia Treated With Deep Brain Stimulation[J]. Biol Psychiatry, 2016, 80(8): e69-e70.
205. CASCELLA N, BUTALA A A, MILLS K, et al. Deep Brain Stimulation of the Substantia Nigra Pars Reticulata for Treatment-Resistant Schizophrenia: A Case Report[J]. Biol Psychiatry, 2021, 90(10): e57-e59.
206. VILELA-FILHO O, RAGAZZO P C, CANEDO D, et al. The impact of subcaudate tractotomy on delusions and hallucinations in psychotic patients[J]. Surg Neurol Int, 2021, 12: 475.
207. GALKIN M V, ZAITSEV O S, GOLANOV A V, et al. Gamma Knife capsulotomy for correction of obsessive-compulsive symptoms in a patient with schizophrenia: Case report[J]. Prog Brain Res, 2022, 272(1): 23-31.
【引用格式】中国医师协会精神科医师分会精神疾病外科治疗联盟,中国医师协会神经调控专业委员会. 中国精神疾病外科治疗专家共识[J]. 中国神经精神疾病杂志,2024,50(7):385-402.
【Cite this article】Chinese Medical Doctor Association Branch of Psychitrists League of Psychosurgery,Chinese Medical Doctor Association Branch of Neuromodulation.Chinese expert consensus on neurosurgical treatment of psychiatric disorders[J]. Chin J Nervous Mental Dis,2024,50(7):385-402.
DOI:10.3969/j.issn.1002-0152.2024.07.001
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言














感谢您的分享
17
#精神病学# #神经外科学# #神经调控# #难治性精神疾病#
23