Cancer Immunology Research:发现靶向PD-L1的癌症免疫治疗新途径
2019-03-30 不详 中山大学生科院
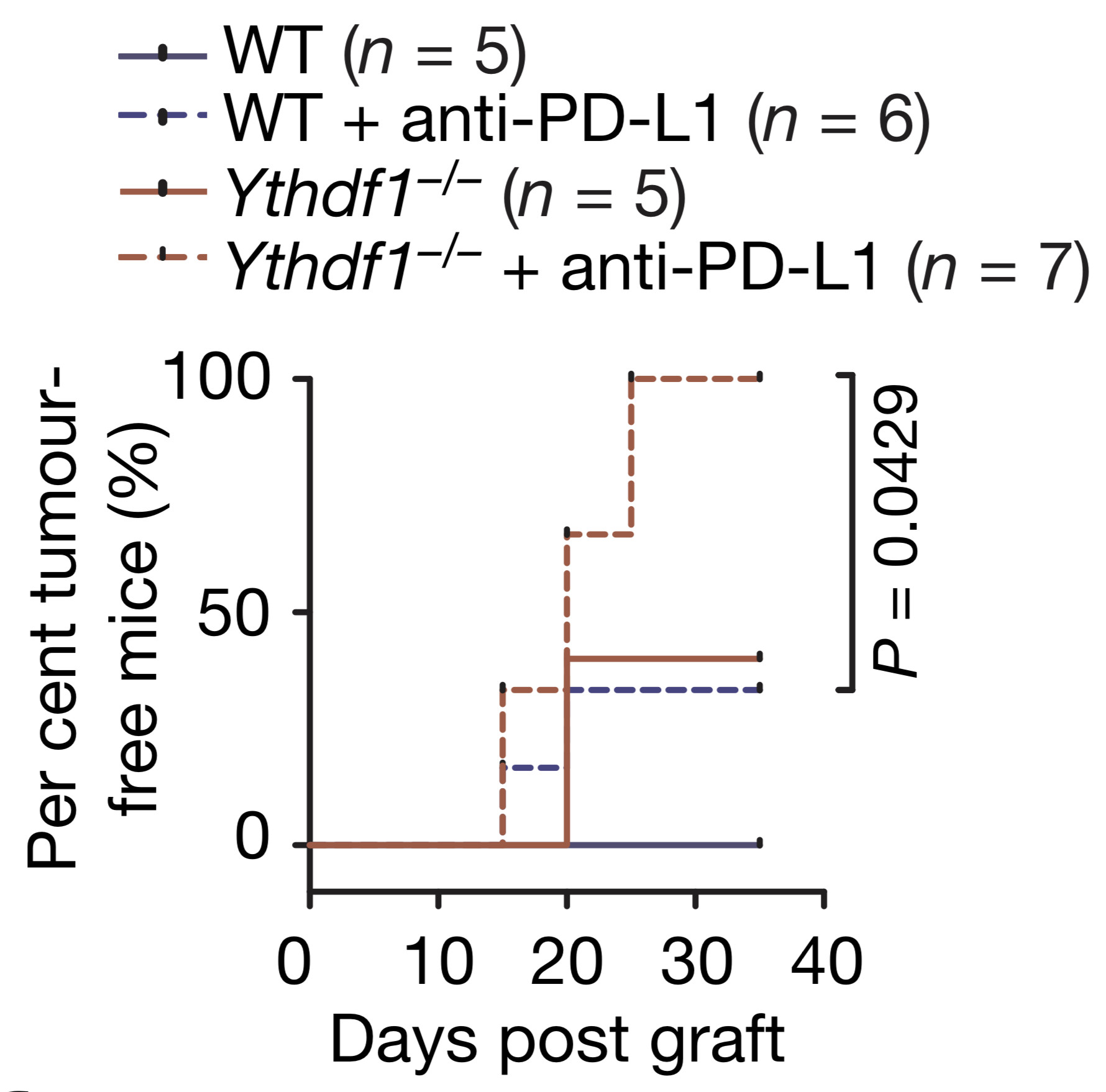
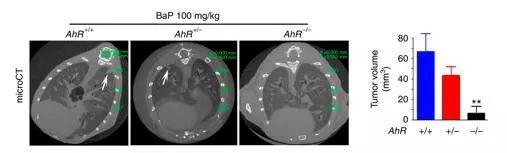
近期,美国癌症研究协会(AACR)旗下重要杂志Cancer Immunology Research发表了中山大学生命科学学院赵勇教授课题组的最新研究成果“LIN28/let-7/PD-L1 Pathway as a Target for Cancer Immunotherapy”。论文发现了癌细胞中PD-L1的调节机制,并得到一种能特异性诱导人体自身T细胞杀灭肿瘤细胞的化学小分子,为癌症免疫治疗提

本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言







#新途径#
46
#治疗新途径#
45
#PD-L1#
39
#Research#
40